| Citation: | Xupeng Xue, Xiaogai Ge, Lei Lei, Benzhi Zhou, Mai-He Li. Effects of phosphorus resorption on bioactive phosphorus of different-aged Pinus massoniana plantations[J]. Forest Ecosystems, 2024, 11(1): 100241. DOI: 10.1016/j.fecs.2024.100241 |
Phosphorus (P) is an important nutrient element in the photosynthesis process of plants. The effect of stand age on P nutrient dynamics in subtropical plantations is unclear. In this study, the mechanisms of above- and belowground P cycling in subtropical P. massoniana plantations of different stand ages (5, 9, 19, 29 and 35 years) were investigated. The percentage of metabolic P in leaves exhibited an initial rise and subsequent decline with stand age of P. massoniana throughout the growing season, culminating in a metabolic P proportion of 34%–68% in litter. During the non-growing season, the main change in the P components of P. massoniana fine roots was a transition from metabolic P to residual P. Relative to during the growing season, soil ligand P fractions decreased by 7%–22% and exchangeable P fractions increased by 0–16% in the non-growing season. Organ P components tended to decrease with increasing stand age, mainly due to the gradual decrease in soil-bioavailable P, a trend alleviated by litter input. The generation of dissolved P by soil phosphatases would limit the reduction in soil-bioavailable P caused by leaf P resorption to some extent. The differences in leaf organ P components between the growing and non-growing seasons are attributed to the allocation of P by the P. massoniana. Overall, the results of this study provide a basis for exploring the mechanisms of soil bioavailable P and soil P enzyme-mediated organ P component allocation in P. massoniana forests. These findings will help improve the management of P-limited P. massoniana plantations to enhance their productivity.
Phosphorus (P) is an important nutrient element in plant photosynthesis (Elser et al., 2007), but the adsorption of mineral P, via leaching and erosion, leads to a decrease in P availability and therefore to P deficiency (Fink et al., 2014; Liu et al., 2018). In response, plants have evolved the physiological and biochemical traits needed to adapt to low-P environments, such as in acidic soils (Lambers et al., 2008). The P cycle in plants contributes to the control of primary production, respiration and organic matter decomposition (Finzi et al., 2011), including P migration or reabsorption among green leaves, litter, fine roots and soil in ecosystems (Li et al., 2022b). Plants are able to increase their P use efficiency (PUE) by efficiently acquiring P from the soil, which can then be preferentially distributed between cells and tissues, thereby overcoming growth limitations and increasing biomass (Han et al., 2022; Veneklaas et al., 2012). For forest stands, mitigating effects associated with declining P acquisition with age can be achieved by implementing conservative PUE strategies (Liu et al., 2023a; Pardon et al., 2017). Through the collaborative processes of bacterial-mediated inorganic P solubilization and external mycorrhizal fungal organic P mineralization, plants take up and recycle accessible P and return it to the soil via the litter (Stuart et al., 2022). P acquisition/utilization strategies may lead to carboxylate release into the rhizosphere, thereby accelerating organic matter decomposition and nitrogen (N) mineralization in the soil by disrupting the stability of aggregates and organic mineral binding (Ding et al., 2021). Exploring P cycling mechanisms in P-deficient soil and the P utilization strategies by plants is pivotal for enhancing our comprehension of nutrient cycling and physiological metabolism activities with forest ecosystems (Han et al., 2022; Jiang et al., 2019).
P fractions from leaves consist of metabolic P, nucleic acid P, lipid P, and residual P (Kulmann et al., 2021). Metabolic P accounts for a small proportion of organic P, but it is important for plant activities such as photosynthetic respiration. Nucleic acid P constitutes the predominant organic P fraction and plays a crucial role in governing the processes of RNA and DNA biosynthesis. Lipid P is found in various cellular components, such as the vacuolar membranes, endoplasmic reticulum, and Golgi apparatus. Residual P content has received limited research attention, mostly in relation to phosphorylated proteins (Cowan, 2006; Suzuki et al., 2010). Leaf P resorption efficiency (PRE) plays a key role in ecosystem functioning and plant nutrient economics (Estiarte et al., 2023), as it guides the allocation of a higher proportion of inorganic P, sugar P, and nucleic acid P to young leaves (Veneklaas et al., 2012; Wang et al., 2019).
The distribution strategies of the leaf P fraction vary with plant species but are aimed at achieving a high PUE. Some plants enhance their PUE to improve productivity and sustain growth, for instance, by increasing metabolic P and decreasing lipid P concentrations (Hidaka and Kitayama, 2011, 2013). Increases in the proportion of nucleic acid P and decreases in those of lipid P relative to total P (TP) have been shown to accelerate protein synthesis and turnover and to improve photosynthetic PUE (Hayes et al., 2022; Wen et al., 2023). As forest age increases, changes occur in the TP of leaves and litter (Xu et al., 2023). Some plants, such as Alnus, have a high P absorption rate in the early stages of forest growth (Sharma et al., 2002). However, the P absorption rate of other plants, such as Cunninghamia lanceolata, increases with the age of the forest (Wu et al., 2023). Fine roots, as organs for plants to absorb soil nutrients, are regulated by P component allocation in their growth and activity (Wang et al., 2018). Although aboveground growth becomes very slow during the non-growing season, belowground growth continues (Lu et al., 2022b).
P contents in plant organs have been found to increase with advancing stand age, but to different varying degrees among plant organs (Li et al., 2022a). The concentrations of soil-labile P fractions decrease with increasing forest age, providing evidence of a decline in P availability over time, which is influenced by soil non-structural carbon (Deng et al., 2024; Zeng et al., 2018). In the later stages of stand growth, microorganisms can control the P components of P. massoniana soil by mediating alkaline phosphatase monoglyceride, where the major influence of arbuscular mycorrhizal fungi is noted (Liang et al., 2020; Liu et al., 2023b). Other studies on how forest age affects plant P have primarily focused on aspects such as microorganisms and soil carbon (C), neglecting the P cycle between soil and plants (Li et al., 2017; Xu et al., 2022).
Soil-bioavailable P drives plant growth, development, and the health of soil ecosystems, through P cycling processes such as adsorption/precipitation and desorption/dissolution (Annaheim et al., 2013; Attiwill and Adams, 1993). Soil-bioavailable P includes organic P (ligand P and hydrolyzed P), which accounts for 30%–60% of total P (Annaheim et al., 2013), and inorganic P (absorbed P and exchangeable P). In leaf P fractions, however, the relative contributions of organic and inorganic P differ among plant species (Gao et al., 2022a). In soils with low P concentrations, plants employ strategies for P recovery and acquisition involving the breakdown of soil organic P through the action of plant-secreted organic acids and enzymes (Darch et al., 2016; Reichert et al., 2022). The assimilation of biological P is influenced by the interplay between the reactivity of soil organic P towards enzymes and the allocation of P fractions within plant leaves. Specifically, phosphatases can impact the partitioning of P fractions in leaves, and vice versa (Gao et al., 2022a; Ushio et al., 2015). PRE is improved by a decrease in the desorption rate of residual P rather than through changes in easily degradable P fractions (Tsujii et al., 2017). This demonstrates the efficient regulatory mechanisms employed by plants to ensure survival in environments with limited P availability.
Pinus massoniana is an important native tree species in China, but the productivity of P. massoniana plantations is significantly reduced by low soil P availability. The P utilization strategies of P. massoniana have been investigated with respect to microbial community structure, metabolic activity, and enzyme activity (Chen et al., 2013; Wang et al., 2014; Yang et al., 2021). Strategies include a transition from “conservative consumption” to “resource consumption”, in which P limitation decreases with stand age (Guo et al., 2023b). An study conducted in P. massoniana plantations revealed that reduced levels of total P and bioavailable P resulted in a higher N:P ratio in green leaves, particularly in plantations younger than 10 years (Liu et al., 2016). In P-poor soils, P. massoniana enhances P availability and mitigates P deficiency through the secretion of acid phosphatase and alteration of its PRE, which varies according to stand age (Tie et al., 2024).
These observations suggest that an understanding of the P cycling mechanisms of P. massoniana could result in enhanced productivity. However, investigations into the mechanism of P cycling from the perspective of organ P components and soil-bioavailable P have been limited. In this study, we analyzed leaf PRE, organ P components, soil-available P, and phosphatase in P. massoniana stands of different ages to explore the mechanisms of aboveground and belowground P cycling as a function of stand age. We hypothesized that: (1) fluctuation of organ P components with stand age is larger in the growing season than in the non-growing season; (2) phosphatase activity is dependent on soil organic P components; and (3) soil-available P, phosphatase, and P resorption are regulated by organ P components. Through this research, we aim to enhance the comprehension of the relationship between plant P and soil P in P-limited P. massoniana plantations, with the goal to improve management practices and subsequently increase plantation productivity.
The study was conducted at the Laoshan Forest Farm (29°33′ N, 119°03′ E) in Qiandao Lake Town, Hangzhou, Zhejiang Province, China (Yang et al., 2016). The study area has four distinct seasons and a subtropical monsoon climate, with an annual average temperature and precipitation of 17.58 ℃ and 1,350 mm between 1980 and 2019 (Xu et al., 2022). The soils in the study area are mainly acidic red soils, with thin layers (30–120 cm) and poor nutrient conditions.
P. massoniana was planted in the study area in 1970. A natural forest of P. massoniana had been cut down for timber, and the logging tracks were subsequently burned and replanted with P. massoniana. Five P. massoniana stands were created at different times (1986, 1993, 2002, 2012 and 2016, corresponding to stand ages of 35, 29, 19, 9 and 5 years at the time of the study in 2021) by the local forestry farm as needed. Their establishment did not follow a fixed time gradient but instead reflected the economic and ecological interests of the forest farm. Three replicates (plots) were established for each stand age, each consisting of a 20 m × 20 m quadrat and separated from the others by 30 m.
Five standard trees (similar diameter at breast height (DBH), no pests) of P. massoniana from each of the five ages were examined during the growing (April–October) and non-growing (November–March) seasons. Samples included 2-year-old leaves, 1-year-old leaves, fine roots, litter, and rhizosphere and non-rhizosphere soil. Two-year-old leaves and 1-year-old leaves were distinguished according to the method of Eimil-Fraga et al. (2015). Specifically, the branches of P. massoniana grow in one round per year; the most vibrant 1-year-old leaves are found at the very front of the tree, while 2-year-old leaves can be found at the second node counting from the top. In addition, litter was collected using litter collectors (1 m × 1 m, placed 1 m above the ground) (Zhang et al., 2019). Fine roots (diameter <2 mm) were excavated with a shovel within 2 m of the base of the tree, along the direction of lateral roots, at a depth of 0–20 cm. They were then thoroughly cleaned with deionized water (Shen et al., 2017). Soil samples from the rhizosphere were obtained by shaking root sections taken from a depth of 2–12 cm in the soil profile until approximately 1 mm of soil remained attached to the roots (Zhou et al., 2022). Plant organ samples were placed in self-sealing bags and were, on the day of sampling (Gao et al., 2022b, 2022c), stored at −80 ℃ until use in determinations of organ P components (metabolic P, nucleic acid P, lipid P, and residual P) and PRE. The 2-year-old leaves and 1-year-old leaves (without damage visible to the naked eye) were selected from the crown. Non-rhizosphere soil was excavated at depths of 0–20 cm at random positions without tree cover within the forest plots. Rhizosphere and non-rhizosphere soil samples were sieved (mesh size >2 mm) and, on the day of sampling, stored at 4 ℃ until being used to determine bioavailable P (soluble P, exchangeable P, ligand P, hydrolyzed P) and soil phosphatases (acid phosphatase, phytase).
Organ P components were divided into metabolic P, nucleic acid P, lipid P, and residual P (Table S1) and determined according to a previously published method (Hidaka and Kitayama, 2013), as follows:
Lipid P: After the petioles and major veins were removed from freeze-dried samples, the leaves were ground in 12:6:1 CMF (Vchloroform:Vmethanol:Vformic acid) and extracted twice with 15 mL CMF, followed by two extractions with 19 mL 1:2:0.8 CMW (Vchloroform:Vmethanol:Vwater). The extracts were washed with 9.5 mL chloroform, and the lower layer was extracted several times to separate lipid P.
Metabolic P: The residue of the lipid P extraction was extracted with 5 mL 85% methanol. The methanol extract was added to a centrifuge tube containing the lipid phase extract and placed in a vacuum for 48 h. After the samples were cooled, the water-soluble substances in the residue were mixed with the methanol extract in the residue. Six rounds of extraction were performed using 10 mL 5% trichloroacetic acid (TCA), with 10 min separating each round.
Nucleic acid P: The residue from the metabolic P extraction was extracted twice with 35 mL 2.5% TCA, followed by a 1 h incubation in a 95 ℃ water bath.
Residual P: The residue after nucleic acid P extraction was defined as residual P.
Organic phosphorus components were measured using the molybdenum blue method and a full-band spectrophotometer at 620 nm (Gao et al., 2022b, 2022c).
PRE determination followed a previously published method (Liu et al., 2016):
| PRE=A1–A2A1×100% |
where A1 represents the P nutrient concentration in new mature leaves (g·kg−1) and A2 is the P nutrient concentration in senescent leaves (g·kg−1).
Bioactive P can be divided into four parts (Table S2): soluble P, exchangeable P, hydrolyzed P, and ligand P. To obtain each one, 0.5 g soil was extracted by adding 10 mM CaCl2, 10 mM citric acid, 0.02 U each of phytase and acid phosphatase, and 10 mM HCl to samples for 3 h with shaking at 180 rpm. The extracts were then centrifuged for 30 min at 25 ℃, 4000 g. The supernatant was removed for analysis at 630 nm using a microplate reader (Infinite 200 Pro, Tecan, Switzerland) and malachite green colorimetry (Wu et al., 2020).
Acid phosphatase was measured using the ligation substrate method, using 4-methylumbelliferone (MUB) (Wu et al., 2020). Phytase activity was expressed as μmol Pi released per min from each gram of fresh sample at 37 ℃ and pH 5.5 (Qin et al., 2022).
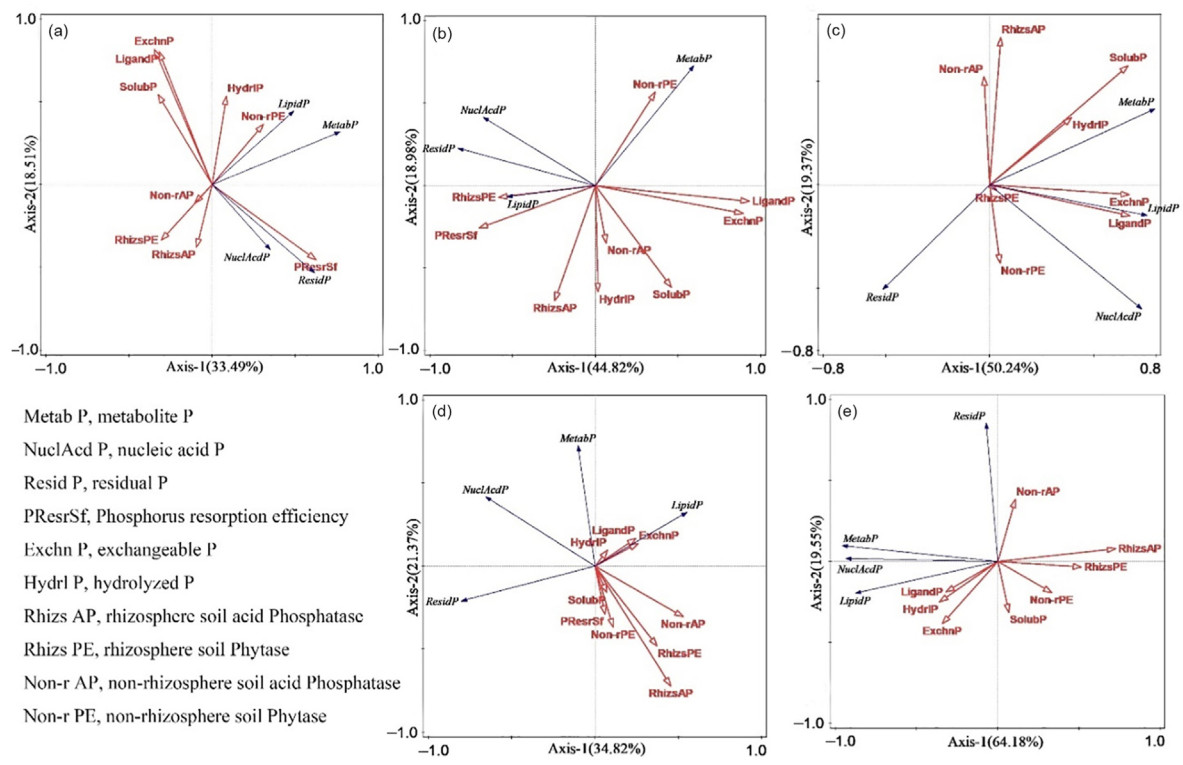
The concentration of each P component in the five P. massoniana stands, leaf PRE, and soil rhizosphere and non-rhizosphere phosphatase activity during the growing and non-growing seasons were evaluated via one-way ANOVA using SPSS 25.0 (IBM Corp., Armonk, NY, USA). The significance level in the statistical tests was defined as p < 0.05. A redundancy analysis was used to evaluate the relationships of organ P components, PRE, and soil phosphatase with soil-available P. IBM SPSS Amos 28 was used to construct structural equation models (SEMs) for organ P components, PRE, soil phosphatase, and soil-available P, using path analysis. All plots were generated using R 4.2.2 (R Coreteam, 2022).
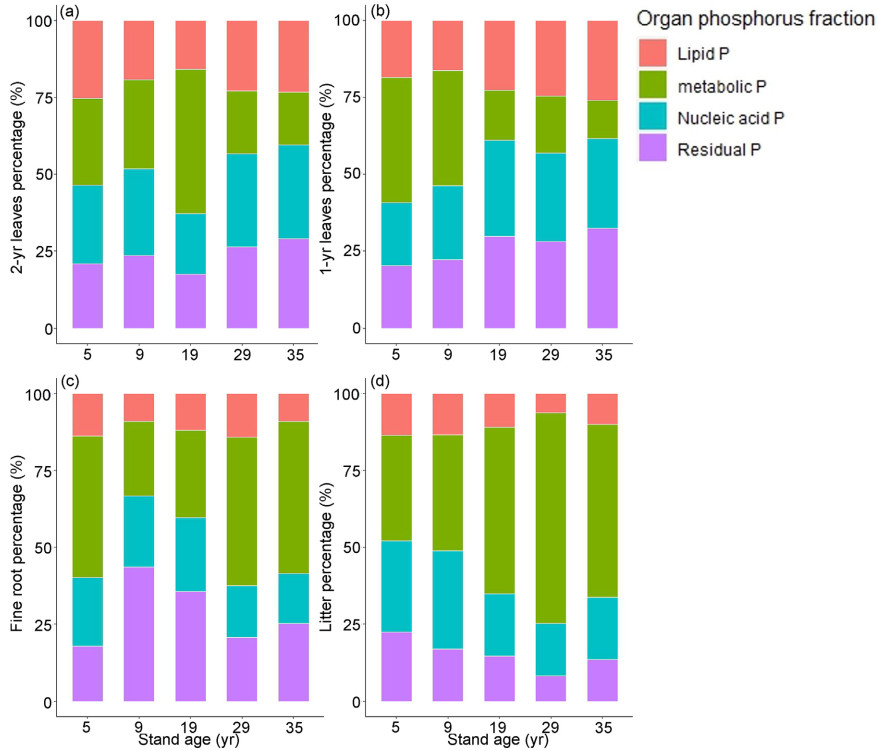
The P fractions of different organs of P. massoniana varied significantly depending on stand age (Figs. S1 and S2). During the non-growing season, the concentration of nucleic acid P in 1-year-old leaves and litter exhibited an initial decline with advancing stand age, followed by an increase, then a decrease once more, reaching a maximum in the 5-year-old stands (for leaves) and 29-year-old stands (for litter) (Fig. S2b). The lipid P concentration in 2-year-old leaves, 1-year-old leaves, and litter demonstrated a pattern of decreasing, then increasing, and subsequently decreasing with advancing stand age, with a maximum reached in the 29-year-old, 5-year-old, and 29-year-old stands, respectively (Fig. S2c). The residual P concentration in fine roots was highest in the 29-year-old stands (Fig. S2d).
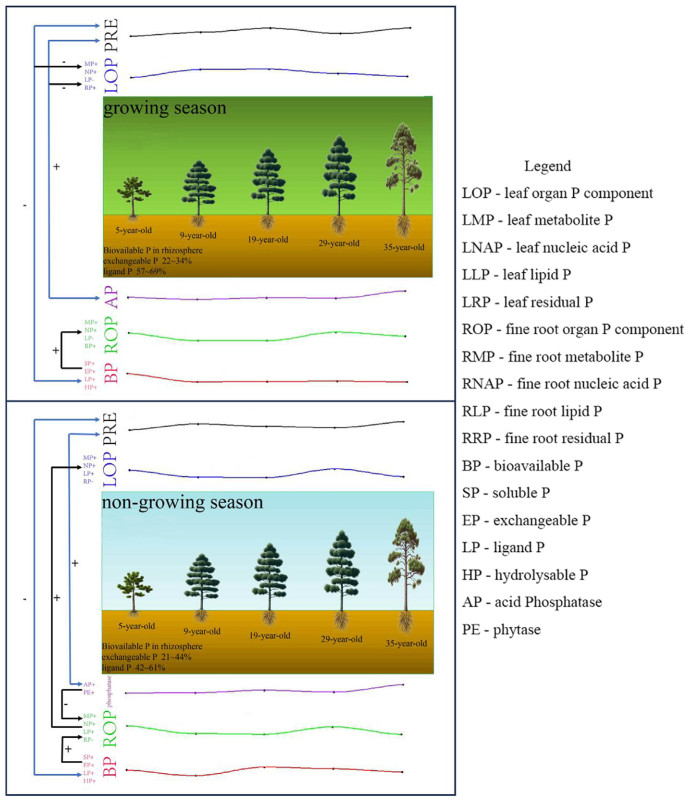
Differences in the proportions of P components in different organs between the growing and non-growing seasons, which exhibited alterations correlated with stand age, were notably more pronounced during the growing season than in the non-growing season (Fig. 1, Fig. 2). The proportion of metabolic P in leaves during the growing season first increased and then decreased with stand age, and the proportion of metabolic P in litter ranged from 34% to 68% (Fig. 1). During the non-growing season, the primary alteration observed in the P components with advancing stand age was a shift from metabolic P to residual P in the fine roots (Fig. 2b).
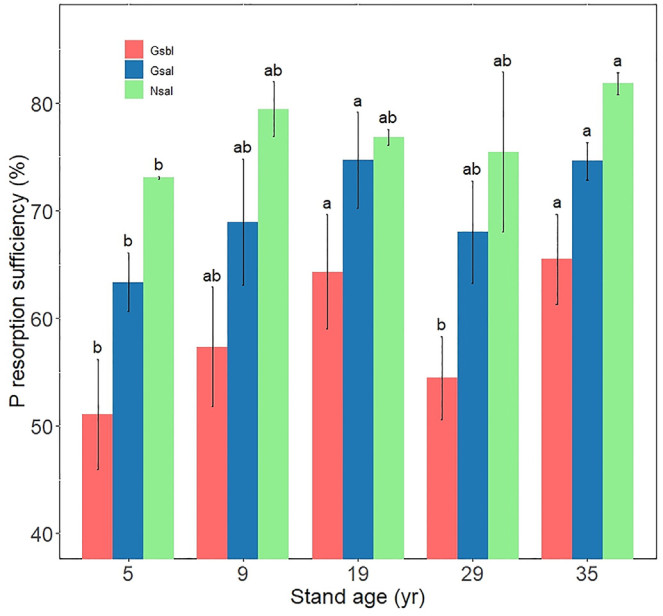
Leaf PRE of different aged P. massoniana stands exhibited a pattern: 2-year-old leaves in the growing season < 1-year-old leaves in the growing season < 1-year-old leaves in the non-growing season (Fig. 3). The pattern followed a consistent trend of initial increase, subsequent decrease, and increase again with advancing stand age (Fig. 3).
The changes with advancing stand age in the concentrations of the four types of bioavailable P (soluble P, exchangeable P, ligand P, and hydrolyzed P) in the soils of the P. massoniana stands were similar, all following a trend of decreasing, then increasing, and finally decreasing again (Fig. S3). During the growing season, the maximum values of soluble P, exchangeable P, and ligand P occurred in the 5-year-old stand, and that of hydrolyzed P occurred in the 19-year-old stand. Compared with the 5-year-old stand, exchangeable P and ligand P in the 9-year-old stand decreased by 87.8% and 81.1%, respectively. During the non-growing season, the maximum values of all four types of bioavailable P were reached in the 19-year-old stand (Fig. S3).
The changes with advancing stand age differed significantly (p < 0.05) among the soil-bioavailable P fractions in the five P. massoniana stands. The proportions of lipid P and hydrolyzed P increased and then decreased in parallel with advancing stand age, while the proportion of exchangeable P showed the opposite trend (Fig. 4). The proportions of the other soil-available P fractions were relatively stable, except in the 9-year-old stands (Fig. 4). Compared with the growing season, the proportion of ligand P fractions decreased by 7%–22% while the proportion of exchangeable P fractions increased by 0–16% during the non-growing season (Fig. 4).
During the growing season, the acid phosphatase activity in the rhizosphere of P. massoniana first decreased and then increased with advancing stand age, while phytase activity followed the opposite trend, with the activities of both enzymes reaching a maximum in the 35-year-old stand. Compared with the 5-year-old stand, acid phosphatase and phytase increased by 94% and 38%, respectively, in the 35-year-old stand. Acid phosphatase activity in non-rhizosphere soil initially decreased and then increased, with a maximum in the 35-year-old stand, whereas phytase activity decreased continuously (Fig. S4). During the non-growing season, acid phosphatase activity in the rhizosphere of P. massoniana first increased and then decreased, before increasing again, whereas in non-rhizosphere soil acid phosphatase reduced first and then increased, with the maximum reached in both cases in the 35-year-old stand. Except for non-rhizosphere phytase activity during the growing season, the activities of both enzymes showed a cumulative increase with advancing stand age (Fig. S4).
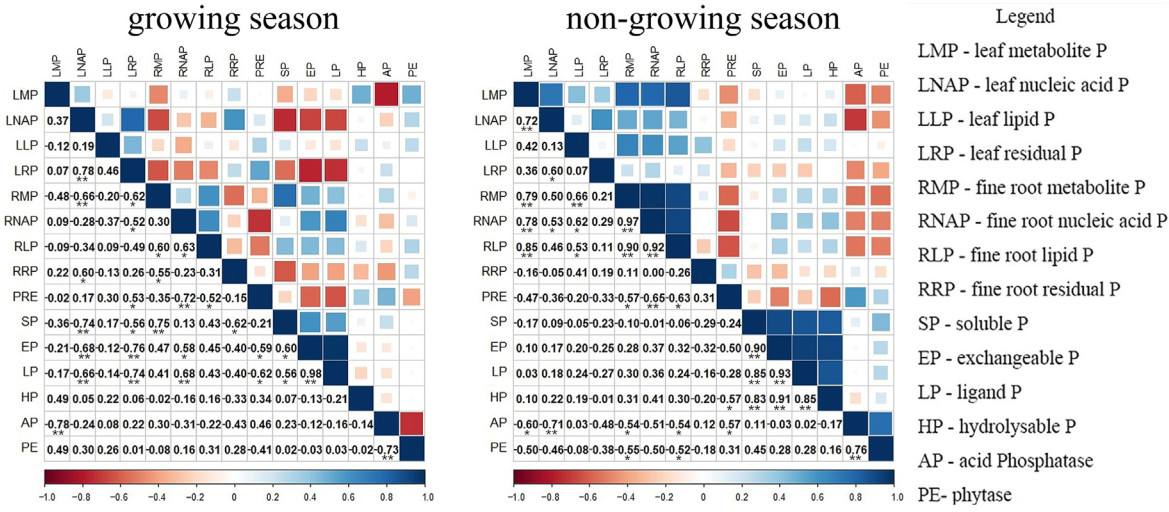
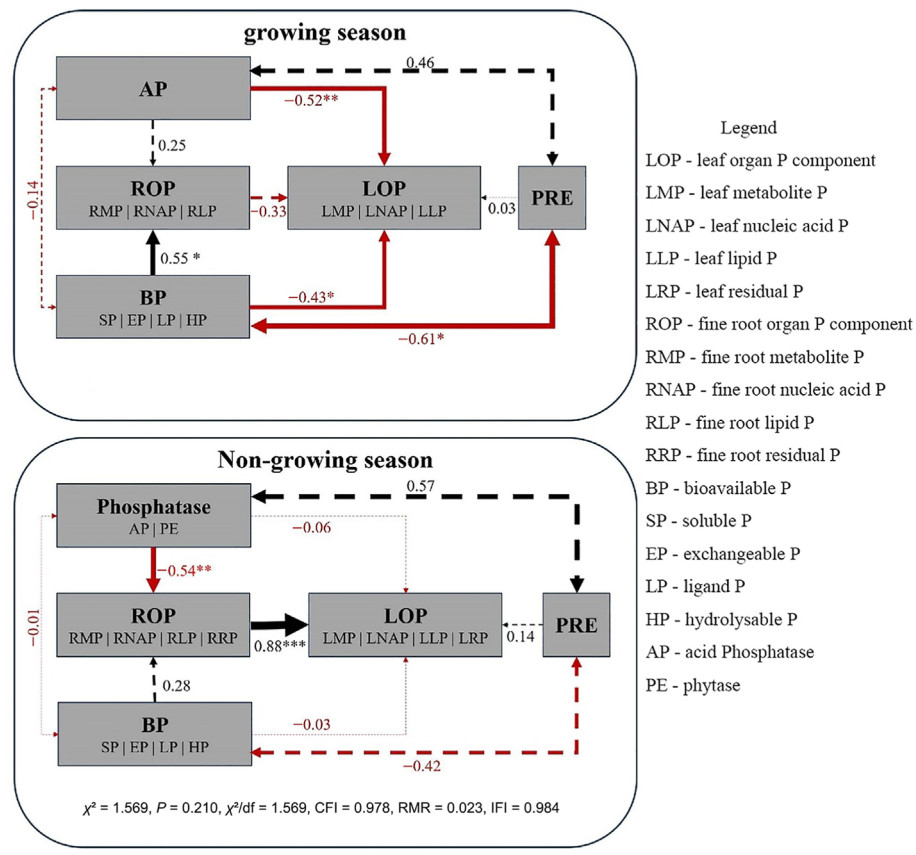
During the growing season, leaf organ P components were mainly regulated by PRE and soil phosphatase, while fine roots were mainly affected by soil P components (Fig. 5). Acid phosphatase activity was negatively correlated with leaf metabolic P (p < 0.01), while soil P components were positively correlated (p < 0.01) with fine root P components (metabolic P, nucleic acid P; Fig. 6). PRE regulated leaf organ P components through its interaction with phosphatase and soil P components, and soil P components significantly affected fine root organ P components (p < 0.05), with a factor loading coefficient of 0.55 (Fig. 7). During the non-growing season, phosphatase and soil P components regulated organ P components (Fig. 6); phosphatase was negatively correlated (p < 0.05) with organ P components, and fine root organ P components were positively correlated (p < 0.01) with leaf organ P components (Fig. 5), which were mainly positively (p < 0.001) regulated by the organ P components of fine roots (factor loading coefficient of 0.88). The organ P components of fine roots were mainly negatively regulated by phosphatase and positively regulated by soil P components (Fig. 7).
Previous studies have involved examining P cycling in old and new leaves, litter, soil and fine roots (Dong et al., 2024; Li et al., 2022b). In our study, organ P components were significantly related to PRE, soil phosphatase and soil P components (Fig. 5). These findings suggest that soil P bioavailability is affected by multiple factors (e.g., organic acids and their concentrations, phosphatase activity), which subsequently impact leaf PRE and the concentrations of various leaf P components (Gao et al., 2022b; Hidaka and Kitayama, 2011, 2013). In P-poor soils, elevated levels of acid phosphatase concentrations are typically observed in the rhizosphere, whereas in P-rich soils, higher concentrations are found in the non-rhizosphere (Hofmann et al., 2016). In our study, the acid phosphatase concentration was much higher in rhizosphere soil than that in non-rhizosphere soil, indicating the important role of P activation in the rhizosphere for the low-P-stressed P. massoniana stands.
Litter input alters the soil C/N ratio, which affects both acid phosphatase and P availability in the soil (Ge et al., 2022; Wang et al., 2022). In our study, the quantity of hydrolytic P exhibited a declining trend with advancing stand age during the non-growing season, followed by an increase, and subsequently a decrease, whereas the inverse pattern was observed for rhizosphere phytase. This suggests that hydrolyzed P is enzymatically decomposed, facilitated by acid phosphatase and phytase, being converted into the orthophosphate form essential for soil organisms (Xu et al., 2022; Yokoyama et al., 2017). With advancing stand age, the enhancement of solubilization of organic bound P is facilitated by the increased dissolution of organic acids (e.g., oxalic acid, malic acid and citric acid; Hou et al., 2018), which could explain the observed decrease in exchangeable P in the older stands.
We also found that bioavailable P was negatively affected by acid phosphatase and the dissolution of organic acids, both of which led to a decreasing trend in the amounts of hydrolyzed P, exchangeable P, and ligand P with advancing stand age. This indicates that low P conditions can improve P uptake by changing the activity of acid phosphatase via the hydrolysis of myo-inositol phosphate, which releases phosphate (Han et al., 2023; Li et al., 2022a; Pang et al., 2015). In the 19- and 29-year-old stands, higher levels of soluble P during the non-growing season than in the growing season might be attributable to a higher demand for soluble P. In contrast, lower concentrations of hydrolyzed P, exchangeable P, and ligand P in the 9-year-old stand during the growing season and non-growing season, and more significant in non-growing season, indicating that although the amount of P required for growth increased, P in the forest floor had not yet begun to return to the soil, resulting in a decrease in the concentration of each P component in the soil (Ma et al., 2007). Typically, the abundant bioavailable P in young P. massoniana stands leads to an increased requirement for P-active enzymes to facilitate the dissolution of bioavailable P (Bi et al., 2021). The reduction of bioavailable P in stands exceeding 19 years of age might have prompted the trees to utilize a substantial quantity of belowground P and to increase the excretion of active P enzymes capable of dissolving bioavailable P (Jarosch et al., 2019).
The content of soil bioavailable P gradually decreased with advancing stand age. On the one hand, the metabolizable P content in P. massoniana leaves increases when they are about to abscise, to enhance PUE (Wen et al., 2023). On the other hand, it compensates for the loss of soil bioavailable P through litterfall recycling (Xu et al., 2022). The increase in litterfall in the later stages of stand development may further improve the activity of phosphatase (Criquet et al., 2004). Ultimately, the decline in soil bioavailable P was alleviated in the oldest stand (Fig. 8).
In the initial phases of P. massoniana stand development, relatively low levels of P are needed, as a considerable amount of P is stored in leaf vacuoles. Previous studies have shown that, with the development of forest stands, decreased availability of P in forest soil leads to a reduction in the metabolic P required for photosynthesis by P. massoniana leaves, resulting in a decline in their photosynthetic rate (Hidaka and Kitayama, 2013; Suriyagoda et al., 2022). By contrast, as the forest develops, the proportion of leaf lipid P gradually increases, indicating an increased allocation of lipid P that inhibits photosynthesis; reduced phospholipid content during the growing season mainly serves to improve PUE, which can help maintain a higher photosynthetic rate (Gao et al., 2022b; Hayes et al., 2022; Niederberger et al., 2019). During the non-growing season, except for residual P in fine root, the changes in P components were consistent and correlated negatively (p < 0.5) with phosphatase activity (Fig. 5).
The concentration of P components in leaves and roots differed significantly between the growing season and non-growing season, which was mainly affected by temperature (Yan et al., 2021). Gene expression is reduced under the low temperature conditions of the non-growing season, to maintain a lower metabolism to cope with low temperature stress (Lu et al., 2022a). In this study, the P concentrations in fine roots initially decreased with stand age, likely driven by the pool of stored orthophosphate (Yan et al., 2019). Further, both P absorbed by fine roots and P allocated to leaf nucleic acid P content decreased with increasing stand age. Thus, a potential bottleneck of aboveground growth would be expected to occur in the later stages of stand development. Fine roots are necessary to increase the uptake of P and other nutrients (Hidaka and Kitayama, 2011). They also regulate the soil P utilization rate via the secretion of organic acids and enzymes. Preceding leaf abscission, P. massoniana reallocates P from old leaves to new leaves, due to limited P availability in the soil. The metabolic P and nucleic acid P in litter steadily diminishes during the non-growing season (Hofmann et al., 2016), until the decomposition of litter eventually leads to the release of nutrients back into the soil, promoting more utilization in the soil (Wang et al., 2021).
In our study, both the PRE and P components were found to be lower in 2-year-old leaves compared with 1-year-old leaves (Figs. S1, S2, 3), suggesting that leaf age predominantly drives the preferential utilization of lipid P and nucleic acid P for PRE (Wang et al., 2019; Zhang et al., 2021). We found that PRE followed the order of 2-year-old leaves in the growing season < 1-year-old leaves in the growing season < 1-year-old leaves in the non-growing season, indicating a lower soil mineral N concentration during the non-growing season than the growing season. The higher PRE of 1-year-old leaves in the non-growing season than in the growing season can be attributed to the lower soil mineral N levels and the higher residual P content observed in 1-year-old leaves than in 2-year-old leaves during the growing season. This suggests that the residual P content plays a role in promoting leaf PRE (Kulmann et al., 2021; Li et al., 2015). PRE regulates P components in leaf organs through the combined action of bioavailable P and active phosphatases (Li et al., 2022a). A negative correlation was observed between PRE and soil P availability (Fig. 7), and following an initial increase PRE decreased over time (Guo et al., 2023a).
The changes in the distribution of P components among various organs can be attributed to variations in soil biological P availability and phosphatase activity (Fig. 6). During the growing season in the plantations, the proportion of metabolic P in 1- and 2-year-old leaves decreases and the proportion of nucleic acid P increases to maintain the growth of new leaves (Gao et al., 2022c). Older leaves are about to become litter, and to enhance photosynthesis of old leaves it is necessary to increase the proportion of metabolic P, which is why litter has a higher proportion of metabolic P than that in new leaves (Gao et al., 2022b). The result that fine roots had a higher proportion of metabolic P during both the growing and non-growing seasons in the later stages of stand development can be explained by the continuous increase in aboveground biomass as stand development progresses, necessitating an increase in root growth rate and nutrient absorption (Hishi et al., 2017). The regulation of the distribution of P components and of PRE in various organs of P. massoniana mainly serves to maintain stability in the above- and belowground P cycle (Fig. 8). In this study, the P cycle process of above- and belowground was analyzed from the perspective of the whole plantation of P. massoniana at different ages. Whether the changes in the P cycle of above- and belowground in forests of different ages are the same needs a large number of samples to confirm, which can be demonstrated in subsequent studies.
Our findings suggest that the interactions of organ P components, leaf PRE, soil P-active enzymes and soil bioavailable P in the aboveground and belowground P cycling system are complex in P. massoniana plantations. During the growing season, younger P. massoniana plantations (<19 years old) necessitate a significant quantity of bioavailable P for growth, as the bioavailable P content is progressively absorbed. Overall, as the stand age of P. massoniana plantations increases, there is a tendency for trees to take up soil bioavailable P during the growing season for leaf growth and development. The demand for P is not high during the non-growing season, and the P components in various organs are relatively consistent with changes in soil bioavailable P. Our findings can provide a theoretical basis for the management practices of P. massoniana plantations and enhance productivity across stand ages in subtropical plantations.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Xupeng Xue: Writing – original draft, Software, Data curation. Xiaogai Ge: Writing – original draft, Resources, Methodology, Data curation. Lei Lei: Software, Data curation. Benzhi Zhou: Software, Methodology, Data curation. Mai-He Li: Writing – original draft, Supervision, Resources, Data curation.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The author would like to thank Professor Ge for her help in the experiment and paper writing. Special thanks to Professor Li and Dr. Melissa Dawes for help editing the manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fecs.2024.100241.