| Citation: | Oliwia Karpińska, Katarzyna Kamionka-Kanclerska, Patryk Czortek, Marcin K. Dyderski, Dorota Czeszczewik. Spatial niche segregation between bird species in the Białowieża primeval forest (NE Poland)[J]. Forest Ecosystems, 2023, 10(1): 100129. DOI: 10.1016/j.fecs.2023.100129 |
Areas characterized by continuous forest cover over extended periods of time are increasingly rare, resulting in a scarcity of trees in present times. Consequently, gathering data in such locations becomes imperative for establishing baseline information regarding modified environments (Basile et al., 2021a,b). One notable example of a remaining temperate, ancient woodland is Białowieża National Park (BNP), situated along the border between Poland and Belarus. The conditions present BNP closely resemble natural settings, providing an exceptional opportunity to investigate organisms in their pristine state and observe their evolutionary adaptations to an environment unaffected by human influence (Wesołowski, 2007). The park's inherent natural characteristics are evident in several primeval forest features, including a remarkable diversity of species, albeit with relatively low population densities. Notably, BNP boasts an impressive avifauna richness, with the occurrence of 90 bird species within a single forest stand—a figure that stands among the highest in European forests (Wesołowski, 2003). Moreover, extensive avifauna studies conducted in BNP over a period spanning 45 years indicate a consistent bird assemblage (Wesołowski et al., 2015).
BNP also showcases other pristine attributes, such as an uneven age structure among trees, a multi-storey stand profile, and a significant abundance of diverse forms of dead wood (Tomiałojć et al., 1984). Furthermore, the park's tree community exhibits remarkable richness, encompassing 26 tree species and 55 shrub species (Faliński, 1991).
Several trees are used by birds more often than others, due to their specific characteristics, determining their habitat-shaping role. These may include food resources (e.g., acorns, seeds, fruits, tree-dwelling invertebrates), nest sites (e.g., cavities or other specific shaped branches suitable for nest placement), various shelter and resting places, etc. (Villard and Foppen, 2018). Tree species are known to be especially valuable for selected groups of organisms. For example, oaks (Quercus spp.) are an important foraging site for the middle-spotted woodpecker (Dendrocoptes medius) (Stański et al., 2021). Aspen (Populus tremula) is commonly used for foraging and nesting sites for other species of woodpeckers (Pakkala et al., 2020), while spruce (Picea abies) is utilized by the three-toed Woodpecker (Picoides tridactylus) (Pechacek and d'Oleire-Oltmanns, 2004) and red crossbill (Loxia curvirostra) (Watson et al., 2009).
Species with similar requirements or close relationships can coexist by dividing and utilizing resources differently. The spatial arrangement of the forest has been recognized as a significant factor for birds that feed on trees (Whelan, 2001). However, birds rely on trees not only for foraging but also for various other life activities, enabling them to occupy distinct ecological niches. Gaining an understanding of resource partitioning patterns among species is crucial for predicting the potential impact of species decline on community and ecosystem functioning (Villard and Foppen, 2018), as well as on stability, resilience, and the concept of functional redundancy (Ricotta et al., 2016). Moreover, studies conducted in sites not transformed by humans are especially valuable, providing a deeper understanding of individual tree species' importance for ecosystem services provisioning (Wesołowski, 2007). Most studies concerning birds' niche separation focus on a few related species or were conducted among one guild (gleaning birds, Alatalo et al., 1987; sunbirds, Riegert et al., 2011; flycatcher species, Koski et al., 2020). Only a few studies concern bird assemblages in the context of their niche separation in forest ecosystems (mixed-species flock, Madagascar forest, Eguchi et al., 1993; primeval beech-fir forest, Slovakia, Adamík and Korňan, 2004). Niche and resource partitioning minimalize interspecific competition (Hood et al., 2021). A narrower ecological niche indicates a higher level of niche specialization and an increased importance of competition between different species. Consequently, organisms may exhibit a diverse taxonomic and functional composition, while specific species abundances remain low. Initial investigations into niche separation among birds in forest habitats revealed that the size of bird populations is constrained by food availability, and different species occupy distinct ecological niches, challenging the previously held belief of competitive exclusion (MacArthur, 1958). Additionally, experimental findings indicate that interspecific competition influences foraging site selection among sympatric tits and goldcrests (Alatalo et al., 1985). In tropical forests, birds also partition their niche by selecting preferred foraging locations or specific branches for lekking. These variations in bird positioning can be influenced by vegetation structure or light availability (Walther, 2002). However, the scarcity of temperate primeval forests has limited the confirmation of this theory in such environments.
This study aimed to analyze the general pattern of niche usage of trees by the bird assemblage during the breeding season. The second aim of our research is to determine if tree species affect the sharing of space in an assemblage of birds and their level of specialization. The background of our research is established by three ecological concepts, i.e. niche partitioning (the division of ecological niches, it refers to the process in which different species or groups of species divide environmental resources to avoid competition and minimize overlap) (Pau et al., 2011); foundation species (a key species that plays a fundamental role in shaping and maintaining an ecosystem, it is a species that creates the physical structure of the environment, providing habitat for many other organisms) (Ellison, 2019); habitat filtering (the process in which specific habitat characteristics influence the occurrence and distribution of species, as a result of this process, certain species are preferentially selected and adapted to specific environmental features, while others are excluded) (Pau et al., 2011).
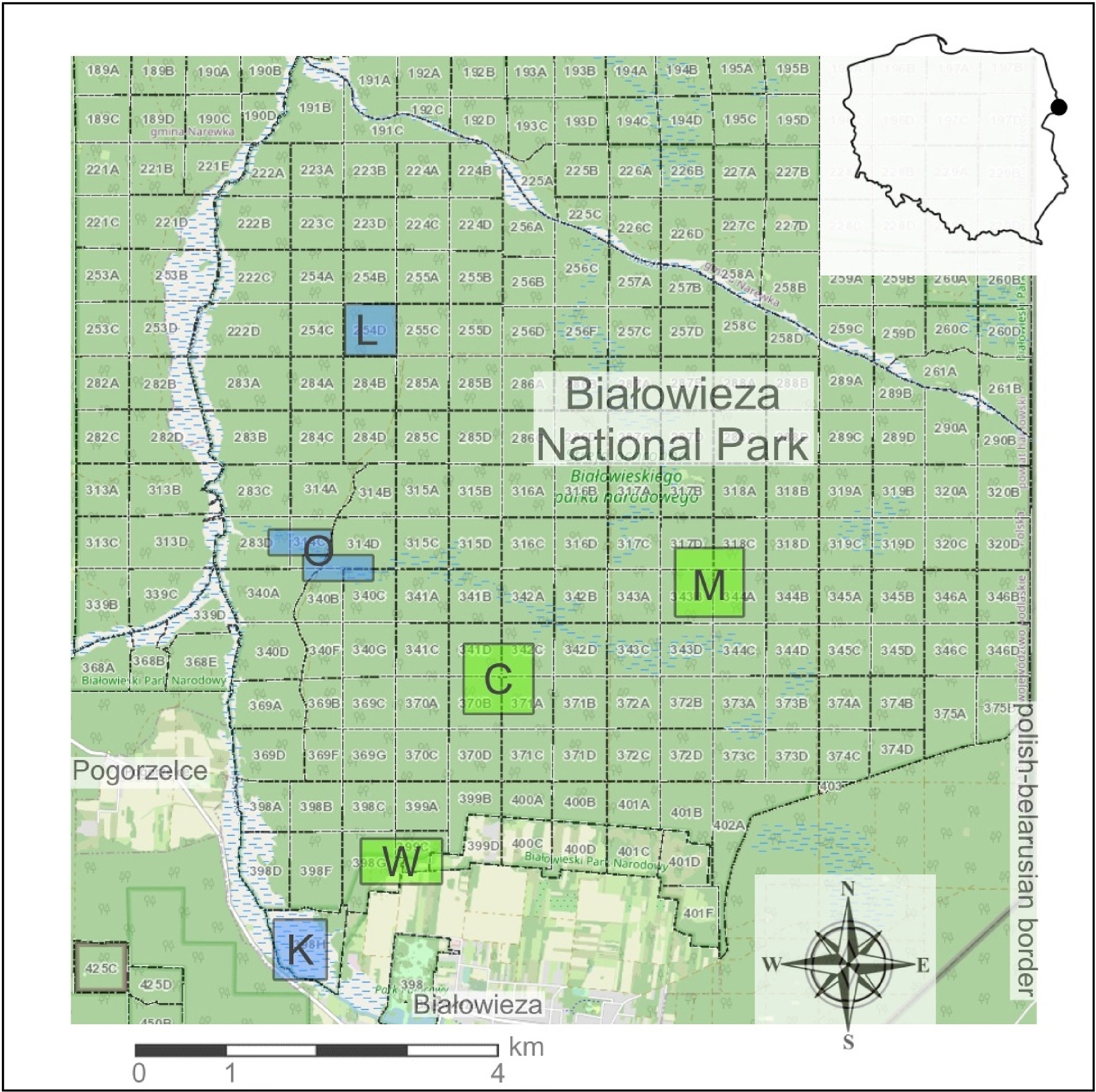
The Białowieża Forest is situated along the Poland-Belarus border, spanning between 23.52° and 24.35° E longitude and 52.48° and 52.95° N latitude (Fig. 1). It stands as the sole remaining lowland temperate forest in Europe. The preservation of its primeval character and vast expanse can be attributed to centuries of protection, initially as a royal and later as a tsarist hunting site. Human impact on the Białowieża Forest has been minimal due to its secured status.
The most well-preserved section of the Białowieża Forest, located on the Polish side, was designated as the Białowieża National Park (BNP) in 1921. Encompassing an area of 105.2 km2, BNP exhibits a natural origin and possesses numerous characteristics synonymous with primeval forests, including a high species richness and diversity of trees, an uneven age structure, a multi-storey canopy, and an abundance of various forms of dead wood (Tomiałojć et al., 1984). The forest comprises trees of varying ages, some attaining sizes unparalleled in other regions. Three main forest types can be distinguished within BNP: oak-lime-hornbeam forest, ash-alder forest, and spruce-pine forest.
The prevailing conditions within BNP closely resemble those found in natural environments, enabling the study of organisms within primeval conditions and facilitating observations of their evolutionary adaptations to an environment unaffected by human impact (Wesołowski, 2007). We conducted a study in BNP in two types of deciduous forests, on six sample plots (three located in lime-oak-hornbeam, called W, M, C, and three located in ash-alder forest called K, O, L). Each of them has 16 randomly chosen observation points, separated by min. 200 m (96 points in total). During the two years of the study, 1,623 observations of individuals belonging to 48 bird species were collected. The total number of collected records was comparable within two habitats: lime-oak-hornbeam plots (W = 382, C = 325, M = 319), ash-alder (K = 370, O = 412, L = 298).
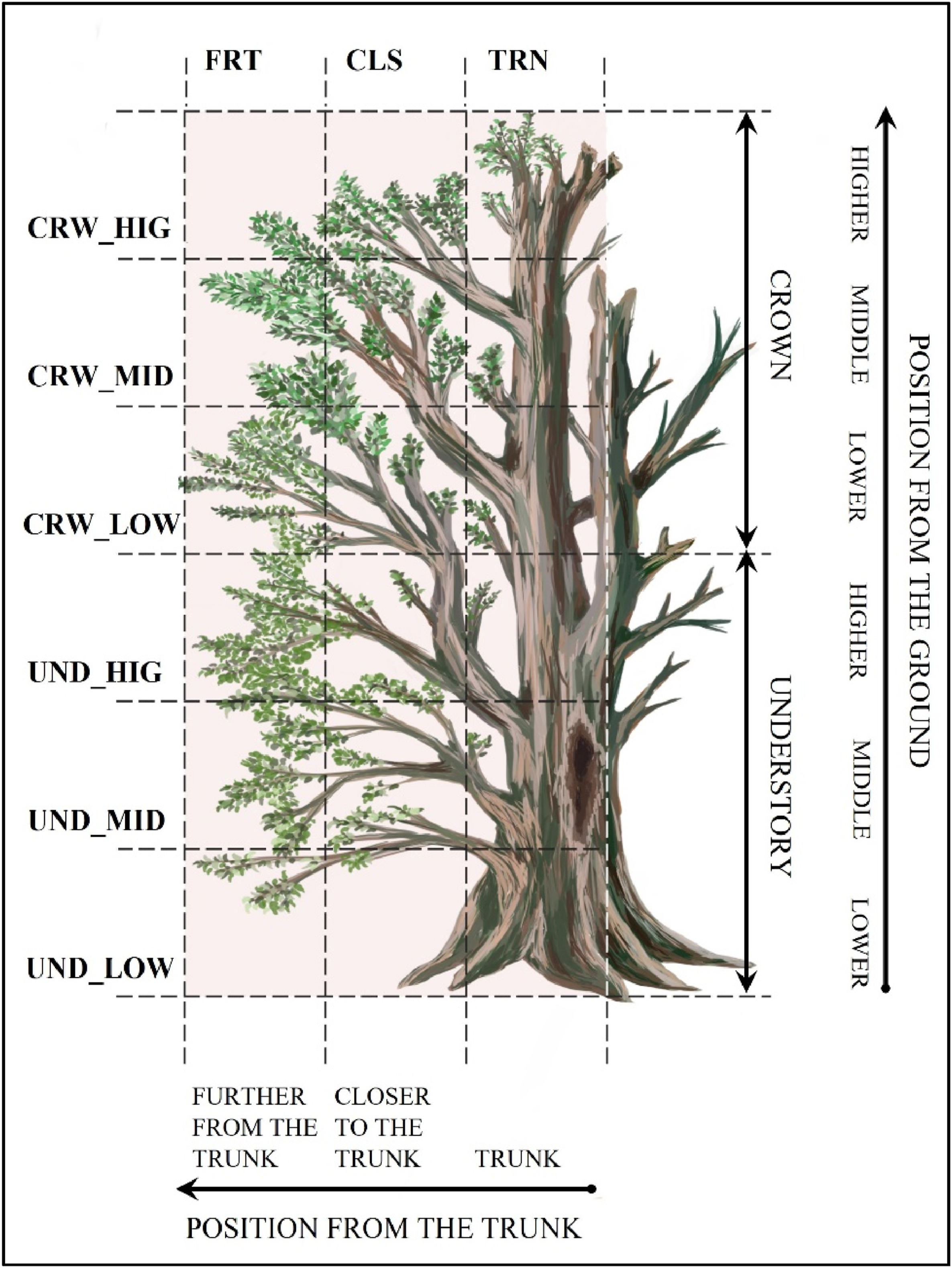
Surveys were conducted in 2020 and 2021 in the breeding season (the last days of April, as well as the whole of May and June). Direct observations of birds at each point were made four times in each breeding season (one morning and one evening during the early season, in late April, early May, and also, one morning and one evening in the late season in July). Morning checks lasted from sunrise to 9 a.m. and evening checks were for the 2 h before sunset. A total of 768 unique visits were recorded over two years, resulting in a total of 192 observation hours. Observations at the points were conducted during optimal weather conditions (windless and rainless). An observer spent 15 min at a point, watching all identifiable birds in sight and using 10 × 50 binoculars. Once an individual was identified to a species, the location where it was first spotted was recorded. This study focused only on locations associated with standing trees, birds in flight, or on other forest structures were excluded. The position (i.e., location on the tree) of an individual was described using a sectoral partitioning form. Forms were created based on the methodology of MacArthur (1958), adapted to broadleaved, primeval forest conditions. Each tree was subdivided into 18 sectors, with a distinction of position from the trunk and position from the ground. Positions from the trunk were divided into three categories: trunk, closer to the trunk, and further from the trunk. Positions from the ground were divided into six categories: low understory, middle understory, high understory, low crown, middle crown, and high crown (Fig. 2).
We conducted all statistical analyses using R version 4.2.0 “Vigorous Calisthenics” (R Core Team, 2022). Instead of traditional methods commonly used while evaluating the statistical significance of the results obtained (e.g., P-values of pairwise comparisons or R-squared determination coefficients undermined by small sample sizes), in our study we focused more on effect sizes. This allowed us to formulate conclusions supported more by biological effects, as well as to avoid omitting results of potentially high ecological importance (Nakagawa and Cuthill, 2007; Wasserstein and Lazar, 2016).
To explore the interactions of birds with tree sectors regarding all tree species and each particular tree species we constructed seven quantitative interaction matrices (networks) based on the frequency of birds on sectors (using the bipartite::plotweb() function; Dormann et al. (2008). We used these seven networks to assess the degree of complementary affinity (specialization) of bird species assemblages to tree sectors regarding all trees and each of six tree species through calculation of the Kullback-Leibler distance d′ index (using the bipartite::specieslevel() function; Dormann et al. (2008). Following Blüthgen et al. (2006a, 2007), d′ index is defined as a deviation from the expected probability distribution, assuming that all bird species interact with sectors in proportion to their observed frequency totals:
| di=c∑j=1(p′ijlnp′ijqj) |
where, di is the non-normalized species specialization index, c is the number of tree sectors, p′ij is the proportion of interactions divided by the sum of i species performances, and qj is the sum of interactions with particular tree sector j divided by the total number of interactions in the matrix. Because the d′ index is normalized di, it is calculated as follows:
| d′=di−dmindmax−dmin |
Index d′ overcomes issues linked with potential asymmetric specialization and indicates the degree of specificity in the species' realized niche (Blüthgen et al., 2006b; Blüthgen, 2010). Index d′ ranges from 0 to 1, where values closer to 0 indicate higher generalization of species (and lower specialization level), and values closer to 1 indicate higher specialization of species to a particular tree sector. Based on the same seven interaction networks, we calculated the resource range RR index (using the bipartite::specieslevel() function; Dormann et al. (2008), which we used for an estimation of the bird species assemblages specificity to particular tree sectors based on the fraction of the space utilized by bird species with a non-zero performance:
| RR=R−rR−1 |
where R is the length of vector containing interaction strengths of the species under consideration and r is the number of non-zero elements within the vector R (Schoener, 1989; Poisot et al., 2012). RR index is a normalization of the total number of links established by a focal species to the particular resource (tree sector), and ranges from 0 to 1: values closer to 0 indicate that almost the entire volume of a particular resource is exploited by the species and values closer to 1 indicate that hardly any of the entire volume of a given resource is utilized by species. The RR index encompasses not only the availability of resources but also takes into account the extent of utilized space. Therefore, in our study, we opted to employ the RR index as an indicator of the spatial range of available resources – adopted terms RR spatial resource index.
To examine how the position of tree sectors from the ground (lower, middle and higher understory, and lower, middle and higher trunk) and from trunk (at the trunk, closer and further from the trunk) influence the mean d′ species specialization index and mean RR resource range index (calculated using bird species occurring on particular sectors independently from particular tree species), we performed two generalized linear models (GLMs; one GLM per index) assuming a beta distribution of the dependent variable (glmmTMB::glmmTMB() function; Brooks et al. (2022). We fitted another two GLMs with beta distribution of response variable to explore differences in mean d′ species specialization index and mean RR resource range index amongst six analyzed tree species (one GLM per index). Using the emmeans::emmeans() function (Lenth et al., 2022), we evaluated marginal differences between variants via the application of Tukey posteriori tests with studentized adjustment for multiple hypothesis testing, and model parameters we presented using the car::Anova() function (Fox et al., 2023). Using the betareg::betareg() function (Zeileis et al., 2022), we calculated the pseudo-R2 coefficient for each GLM. We visualized differences in d′ species specialization index and mean RR resource range index amongst the position of sectors and tree species using the marginal mean plots and heatmaps.
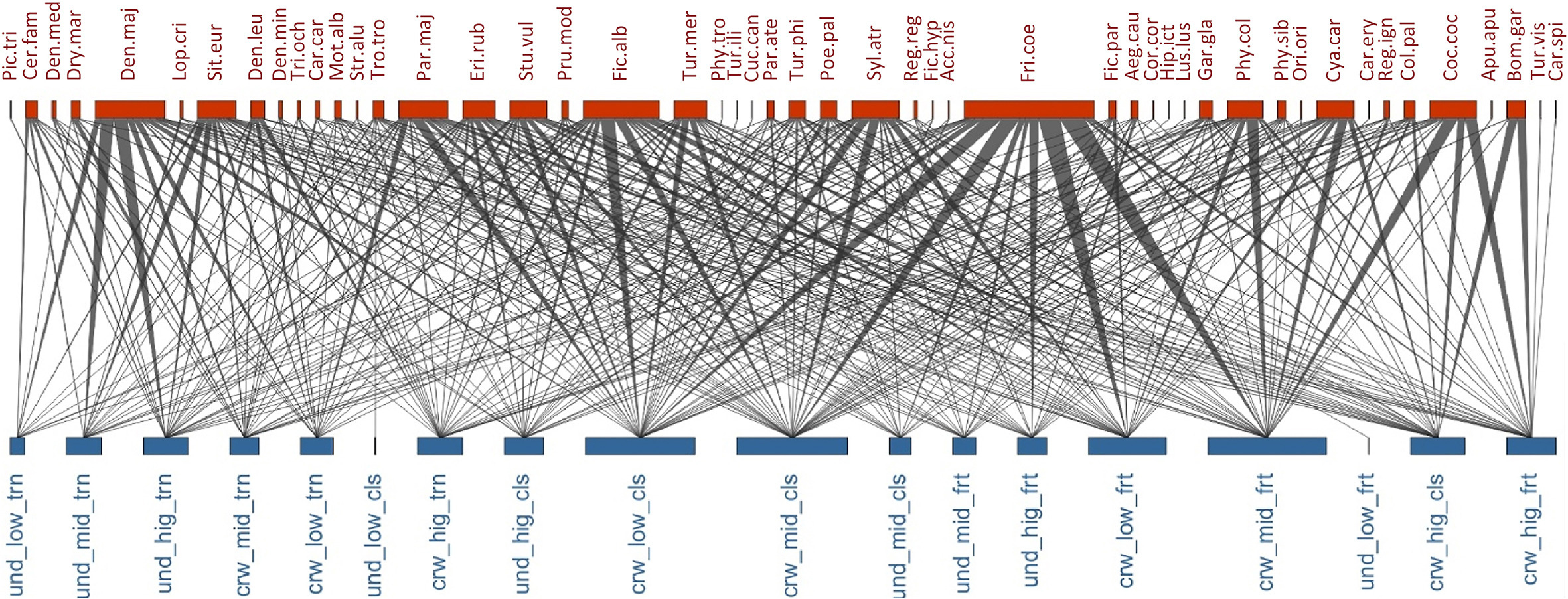
Overall, we identified 345 interactions between 17 tree sectors (we studied 18 sectors, but low understory further from the trunk was never used by birds) and 48 bird species. We observed the most numerous interactions in sectors located in the middle and lower crown closer to the trunk (35 and 29 interactions, respectively), middle crown further to the trunk (26 interactions), as well as sectors located in the higher crown at the trunk, closer to the trunk and further from the trunk (23–25 interactions; Fig. 3). The number of links varied between each tree species. We found the most numerous interactions in alder (209), hornbeam (163) and spruce (135), while lime (121), maple (73), and oak (59) revealed a smaller number of connections; Supplementary Material Fig. S1).
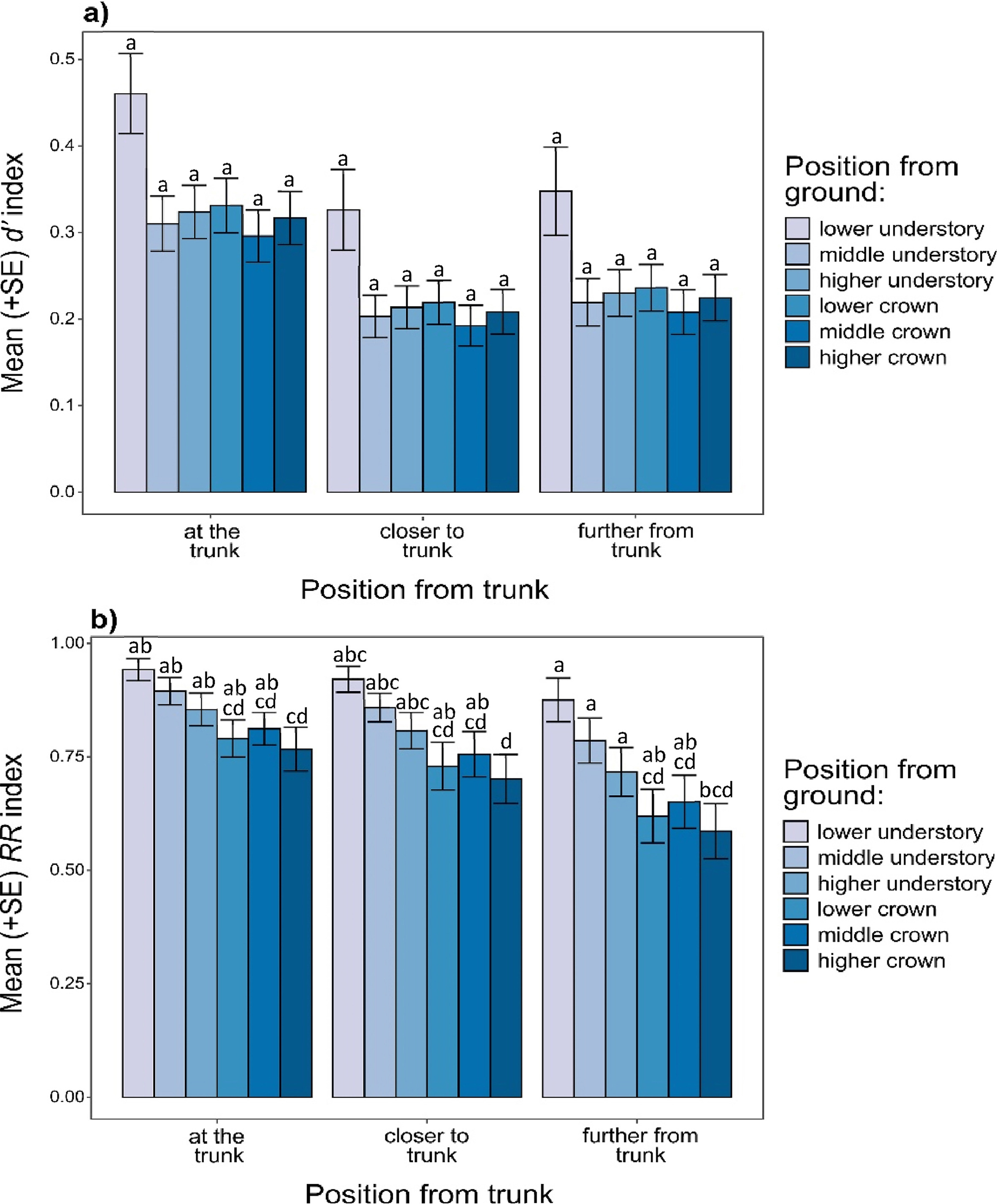
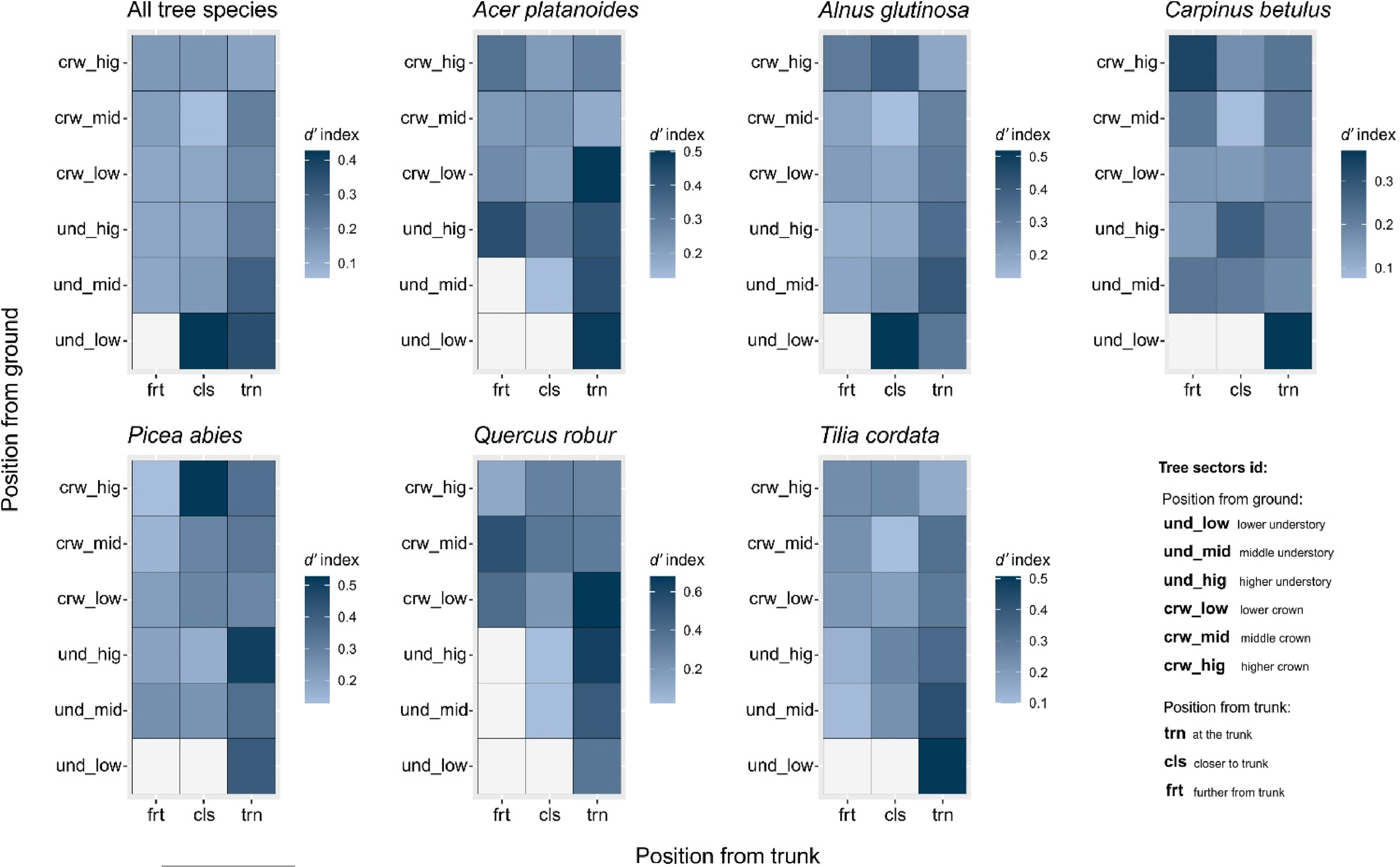
Species specialization index d′ reached the highest values in the lower understory (position from the ground) on the trunk position reaching 0.45. Lower values we observed on the trunk at higher crown and understory level (0.34), at lower crown level (0.34), further from the trunk position at lower understory level (0.34), on the trunk at middle crown and understory level (0.32), and also closer to the trunk at lower understory level (0.31). We observed low values of bird specialization index further from the trunk at the middle crown and understory level (0.23), and further from the trunk at the lower crown level (0.26), higher crown (0.24) and understory level (0.25), and also closer to the trunk at lower crown level (0.23). The position closer to the trunk at higher and middle levels reached the lowest values of the d′ index (higher crown and understory −0.22; middle crown and understory −0.20 and 0.21), (Fig. 4a). ANOVA of GLMs with beta distribution explaining differences in d′ species specialization index among the position of tree sectors from ground and trunk (independently from tree species), is presented in Table 1.
| Response | Variable | X2 | df | P | Pseudo-R2 |
| d′ index | Position from trunk | 22.240 | 2 | <0.001 | 0.229 |
| Position from ground | 10.598 | 5 | 0.059 | ||
| RR index | Position from trunk | 12.215 | 2 | 0.002 | 0.155 |
| Position from ground | 18.592 | 5 | 0.002 | ||
| d′ index | Tree species | 13.315 | 6 | 0.038 | 0.108 |
| RR index | Tree species | 31.151 | 6 | <0.001 | 0.134 |
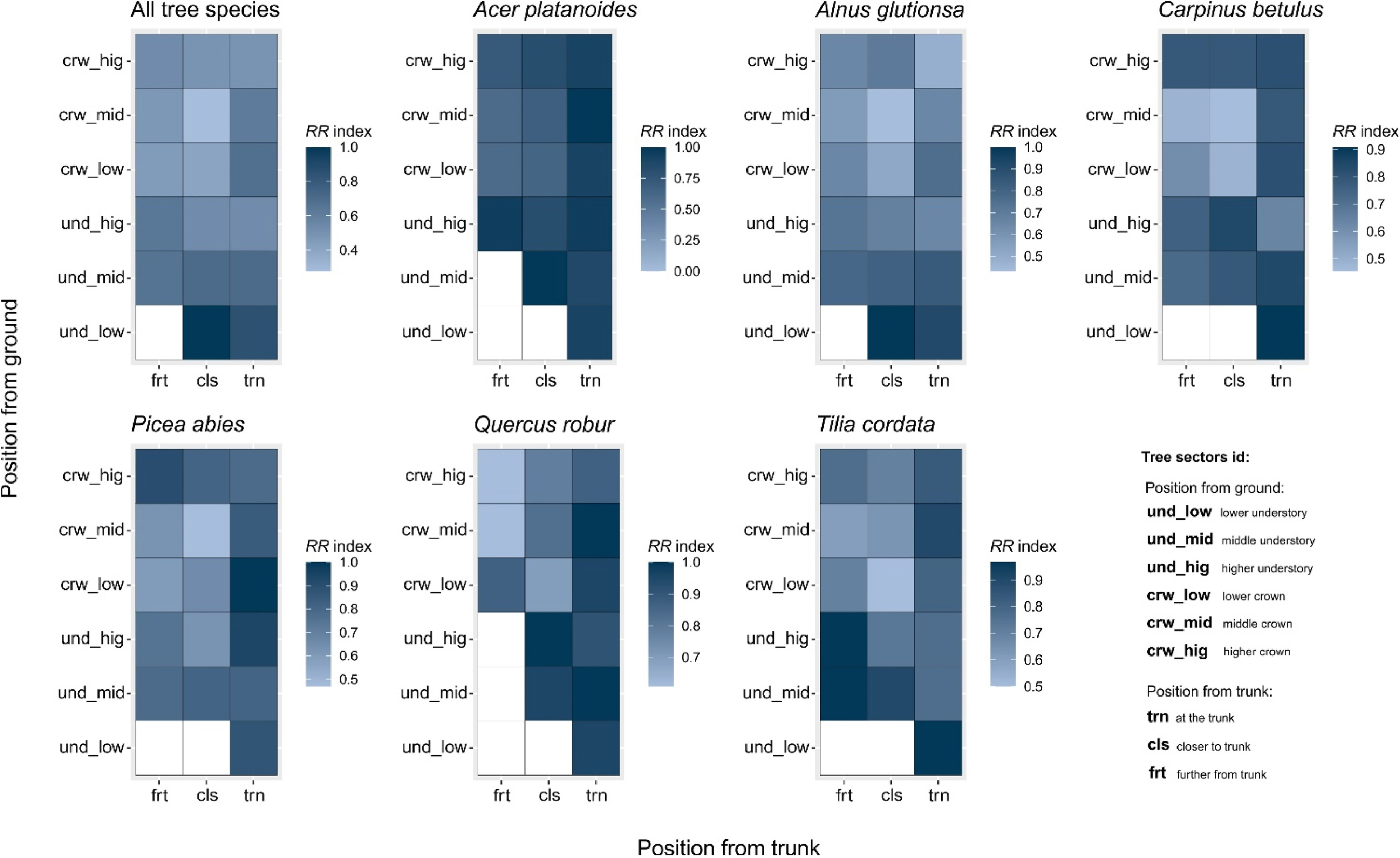
Spatial resource range index RR performed slightly differently in comparison to d′. We found the highest values of RR for the lower understory on the trunk (0.93), middle understory on the trunk (0.92), lower understory closer to the trunk (0.89), higher understory on the trunk (0.88), and middle understory closer to the trunk (0.88). Lower values of the RR index we observed on the middle crown on the trunk (0.84), higher understory closer to the trunk (0.84), lower understory further from the trunk (0.83), lower crown on the trunk (0.82), middle understory further from the trunk (0.81), higher crown on the trunk (0.80), and middle crown closer to the trunk (0.78). The lowest values of the RR index we found on lower crown, both closer (0.76) and further (0.64) from the trunk, higher crown, both closer (0.73) and further (0.61) from the trunk, middle crown further from the trunk (0.68), and higher understory further from the trunk (0.75) (Fig. 4b). ANOVA of GLMs with beta distribution explaining differences in RR spatial resource range index among the position of tree sectors from ground and trunk (independently from tree species) is presented in Table 1.
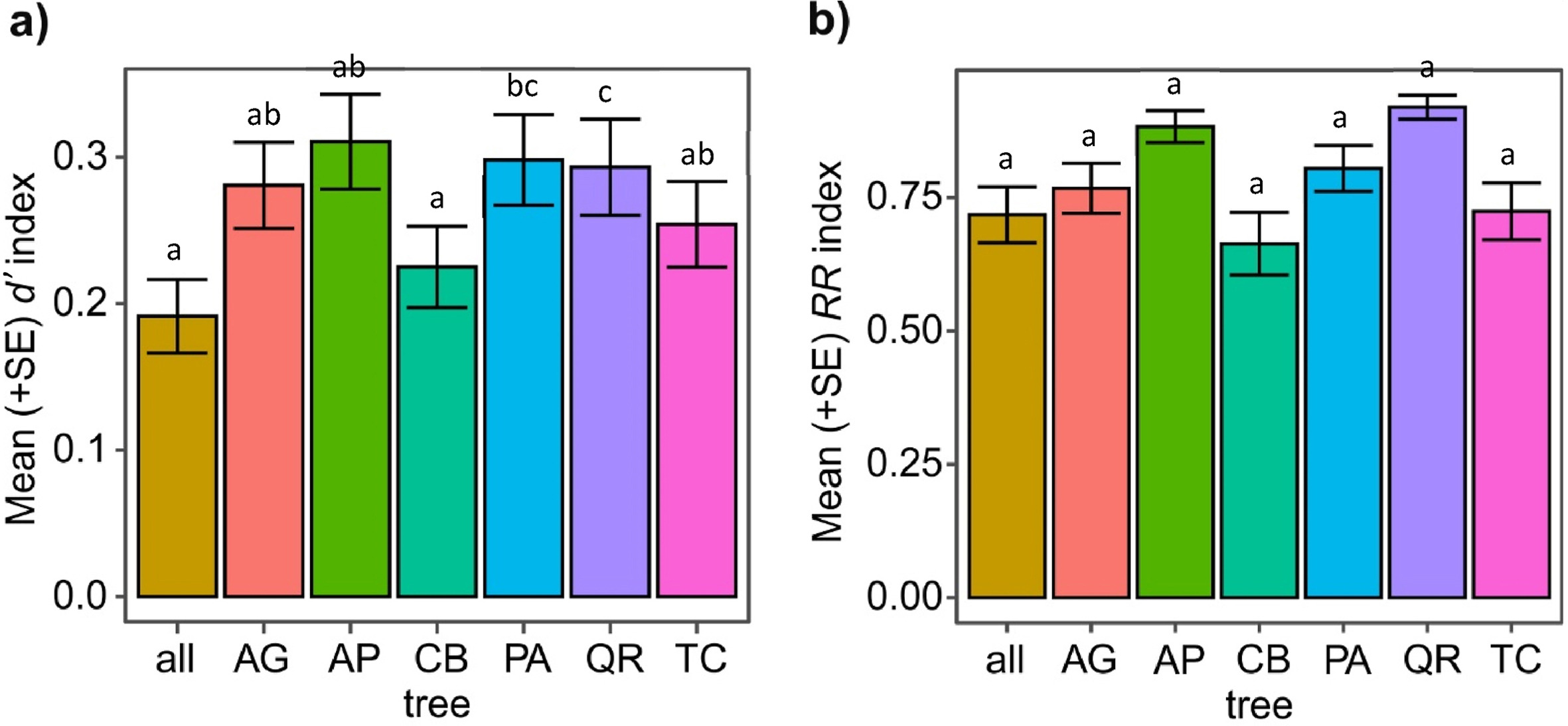
Regarding the tree species, the lowest d′ index was observed in hornbeam. Higher values we observed for lime, whereas birds showed the highest specialization in oak. Medium values were found in the cases of maple, alder, and spruce. (Fig. 5a). At the tree species level, RR index was the lowest for hornbeam, and a little higher for lime, and black alder. The highest values were found in maple, spruce, and oak (Fig. 5b). We also performed values of d′ and RR index for all trees together, to showcase how bird specialization appears in particular tree species within the context of all trees. ANOVA of GLMs with beta distribution explaining differences in d′ species specialization index and RR spatial resource range index among tree species are presented in Table 1.
Considering all tree species together, birds' specialization to a particular sector is homogeneous on the whole tree except the lower part of the trunk and the low part of the understory. The low part of the understory, further from the trunk, was never used by birds (Fig. 6). Regarding the specialization index at the tree species level, we found black alder to be the most equally utilized by birds. We observed the highest specialization index on the trunk and on the low understory, and the high crown closer to the trunk. Hornbeam, spruce, and lime were similarly used by birds, and most of the tree sectors were used equally. The most heterogenous birds specialization were found on maple and oak (Fig. 6).
Heatmaps showing the RR spatial resource range index on each sector of particular tree species display the tree area utilization by birds (Fig. 7), the higher the value of the RR index, the fewer resources the tree sector offers to birds. Considering all tree species together, the spatial resource range RR index on a particular tree sector is almost equal on the whole tree except for the lower part of the trunk and the low part of the understory. We observed that the low part of the understory, further from the trunk, had no useful resources for birds. Especially since such branches were infrequently observed in the forest. Regarding the RR index at the tree species level, we found that out of all tree species, black alder offers its sectors to birds the most equally. We found that spruce offers the most numerous resources for birds in six sectors. Hornbeam was found to have the lowest values of the RR index closer to and further from the trunk, at the middle and low crown, and also on the high understory trunk. In the case of lime, we observed the lowest values of spatial resource range RR index in the low crown sector closer to the trunk. We observed that every maple sector had almost equal low values of the spatial resource range RR index. Oak had the lowest values of the RR index regarding high and middle crown further and closer to the trunk and also in low crown closer to the trunk (Fig. 7).
The low understory sector located closer to the trunk exhibited the lowest utilization by birds, as indicated by the high spatial resource range (RR) index. Additionally, the low understory sector further from the trunk was never utilized by birds. There could be multiple reasons why birds find these locations unattractive. For instance, the low understory sectors typically lack dense foliage, which is known to have a positive correlation with the abundance and diversity of birds (MacArthur and MacArthur, 1961). The presence of poor foliage in the lower understory sectors of trees likely leads to a reduced abundance of invertebrates that typically inhabit the leaves. These lower parts of trees are often shaded, resulting in the absence of flower development, which serves as a food source for certain herbivorous species (e.g., Tomiałojć, 2005). Moreover, the reduced sunlight availability in these areas may result in decreased insect activity (Mueller et al., 2016). Additionally, it is worth noting that the occurrence of such branches in the lower understory sectors is rarely observed (personal observation). Birds exhibit significant specialization in relation to sectors closer to the trunk of the tree. Tree trunks, being thicker and characterized by broader and more sculpted bark, potentially provide less readily available food compared to other parts of the tree. However, tree trunks are commonly utilized by several bird species classified as bark feeders and cavity-nesters (Wesołowski et al., 2015). These birds exhibit morphological adaptations such as long and sharp claws, zygodactyl feet, a strong beak, stiffened tail feathers, and a long sticky tongue, enabling them to exploit the narrow niche available on the trunk (Wesołowski, 2011). The presence of different types of tree cavities further contributes to the utilization of trunks by specific cavity-nesting bird species. Each bird species has specific requirements regarding cavity localization and dimensions, which indicates resource partitioning within this group of birds (Wesołowski and Martin, 2018).
Birds exhibit the least specialization for the middle part of the tree crown. This particular location is characterized by the highest density of leaves (Walther, 2002). Other vegetation features that significantly influence bird diversity include foliage volume, density of vegetation cover (Karr and Roth, 1971), and the percentage of canopy cover (Jayson and Mathew, 2003). In Białowieża National Park's deciduous forests, approximately 90% of birds adopt an insectivorous diet during the breeding season, with more than half of them being crown foragers (Tomiałojć et al., 1984). In other European temperate deciduous forests, birds commonly forage in both tree crowns and on the ground vegetation (Tucker and Evans, 1997). Although the ground vegetation was not specifically included in our study, occasional observations of birds foraging on the ground were noted in Białowieża National Park (unpublished data). In tropical Indonesian forests, ground foraging birds tend to occupy dark and cool areas that are challenging to find outside the forest interior. The pattern of space utilization by birds that we observed in BNP appears to resemble that found in Indonesian tropical forests, where the middle canopy is the most frequently utilized part of the tree, while the ground and emergent layers are less frequently utilized (Dinanti et al., 2018). In our study, we examined not only foraging but also other life activities of birds, and we found that the middle parts of the crowns were most frequently utilized. Besides habitat availability and species requirements, interspecific competition serves as one of the drivers influencing bird species composition (Graves and Rahbek, 2005). Basile et al. (2021a,b) provide evidence that the presence and number of species in a particular area are influenced by both specific environmental factors and the coexistence of other species. However, several researchers suggest that species competition plays a minor role in the pristine Białowieża Forest (e.g., Tomiałojć et al., 1984; Wesołowski, 2007; Czeszczewik and Walankiewicz, 2016). Furthermore, predation and the adaptations of birds to evade predation are crucial factors to consider (Villard and Foppen, 2018). However, these topics were not addressed in the present paper.
Each tree species possesses unique physical characteristics, including wood density, bark structure, branch shape and arrangement, and crown size, which can also vary with the tree's age (Hoadley, 1998). Trees with more diverse and complex structures offer greater opportunities for bird utilization compared to trees with smooth trunks or branches, smaller and higher crowns, or leaves that are inedible to insects (Ghadiri Khanaposhtani et al., 2012). Among the studied sectors, hornbeam and alder were utilized by birds in a more homogeneous manner (Fig. 5). Hornbeam is one of the most abundant trees found in oak-lime-hornbeam stands (Faliński, 1986). Many bird species in BNP most often use hornbeams for nesting, e.g., Marsh Tit (Wesołowski, 1996), Hawfinch (Tomiałojć, 2005), Red-breasted Flycatcher Ficedula parva (Mitrus and Soćko, 2001), Great Tit (Maziarz et al., 2015), Blue tit Cyanistes caeruleus (Wesołowski and Rowiński, 2012), Collared Flycatcher Ficedula albicollis (Walankiewicz et al., 2007), and Robin (Karpińska et al., 2021). Hornbeam trees exhibit distinct characteristics, such as a relatively short and highly branched trunk and a dense crown with numerous thin branches (Boratyńska, 1993). As the tree ages, the branches gradually break, creating cavities that are often long-lasting due to the tree's relatively hard wood (Wesołowski, 2012). The bark of older hornbeam trees, although thin and smooth, thickens and develops numerous cracks (Johnson, 2004). The fauna of invertebrates associated with hornbeams includes dozens of species that inhabit the bark, wood, surfaces of trunks and branches, gaps, and even leaves (Szmidt, 1993). Additionally, older hornbeams often have numerous dead or decaying fragments. These characteristics make nearly every part of the tree attractive to birds.
The black alder is the dominant tree species in ash-alder stands. The breeding bird community found in this habitat is characterized by high density and species richness (Wesołowski et al., 2015). We observed that nearly all the species in this habitat utilized the alder tree extensively. Therefore, alder plays a similar ecological role in ash-alder stands as hornbeam does in oak-lime-hornbeam stands, despite being structurally different. Alder trees have a tall, soaring trunk, and their crown starts at a considerable height above the ground (Faliński and Hereźniak, 1977). Numerous birds in ash-alder stands mostly nest in alders, e.g., the Starling (Wesołowski, 2021), the Hawfinch (Tomiałojć, 2012), woodpeckers (Hebda et al., 2016), titmice (Wesołowski and Rowiński, 2012). However, the number of tree species in BNP's ash-alder forests is much lower (10 tree species) than in oak-lime-hornbeam stands (16 tree species) (Faliński and Hereźniak, 1977), and birds have fewer choices as a result, especially since the second most abundant tree – the ash, is now rapidly dying (Wesołowski, 2021). For example, in the past, in ash-alder stands in BNP, the Starling used to nest most often in ash, but now it nests in alder (Wesołowski, 2021). Ash was regarded as a foundation species in riverine forests. It is an important species for many organisms, e.g., epiphytes, and it is regarded as a biodiversity hotspot (Turczański et al., 2021). In the context of ash dieback, its foundation position in the riverine forest could be taken over by alder, as it was found regarding lichen species (Łubek et al., 2019). Both hornbeam and alder trees are utilized extensively and homogeneously by birds, suggesting that they provide not only abundant food resources but also a wide range of microhabitats and nesting places. This extensive use of these trees may indicate the presence of generalist bird species, but they can also be considered “foundation species” that create and support diverse niches. This leads to niche partitioning, where numerous species occupy narrow niches.
The third tree species that exhibits homogeneous use by birds, and can be considered a foundation species, is spruce. Spruce occurs in various proportions in all types of Białowieża Forest stands. However, in recent decades, there has been a noticeable decline in the number of spruce trees due to frequent outbreaks of the spruce bark beetle Ips typographus, which is associated with climate change and its impact on habitat suitability (Dyderski et al., 2018; Kamińska et al., 2021). As a result, bird species that rely on spruce trees may face the risk of disappearance or modification of their niches. All sectors of spruce trees were found to be used fairly evenly by birds. The structure of spruce is relatively uniform, with a trunk covered in thin and finely cracked bark throughout its length, and a narrow and soaring crown (Johnson, 2004).
The observations in the study indicate that birds were observed in a narrower range of tree sectors when it comes to maple, lime, and oak trees. This suggests that these tree species provide specific resources in certain locations within the forest.
Maple trees in the Białowieża Forest are known to host a variety of epiphytic moss species (Wesołowski and Wierzcholska, 2018). These mosses can provide nesting materials and habitat for certain bird species, such as wrens (Wesołowski, 1983). Maple trees also serve as important nesting sites for Nuthatches (Wesołowski and Rowiński, 2004). Additionally, mistletoe growth on maple trees can create nesting opportunities for hawfinches (Tomiałojć, 2005). Maple wood is known for its hardness and durability, and the bark of older maple trees tends to develop longitudinal cracks.
Lime trees, along with hornbeam, are the second most abundant tree species in oak-lime-hornbeam stands in the Białowieża Forest (Faliński, 1977). However, there are relatively few old lime trees with diameters of up to 200 cm, which have a structure somewhat similar to old oaks. These large lime trees serve as important nesting sites for Nuthatches (Wesołowski and Rowiński, 2004). Younger lime trees are more numerous but have simpler structures compared to the old trees, making only specific sectors of these trees attractive to birds.
On the other hand, oaks in Białowieża National Park (BNP) are sparsely distributed but can reach large sizes, with trunk diameters of approximately 200 cm and heights over 40 m (Faliński, 1977). The most common oak individuals in BNP are old trees with tall, soaring trunks and huge crowns. Oaks also serve as nesting sites and important feeding locations for certain bird species, such as the Middle Woodpecker (Stański et al., 2021).
Indeed, the density and hardness of wood, as well as the physical characteristics of trees, can act as habitat filters, shaping the bird community and reducing the number of species that can effectively utilize specific resources. Trees with dense and hard wood, such as hornbeam, maple, and oak, may offer specialized resources that are not accessible to all bird species. These specialized resources, in turn, can lead to high specialization among bird species, as they adapt to utilize specific niches within the tree habitat. As a result, this specialization may lead to lower taxonomic and functional diversity within the bird community. The reduced accessibility of resources in certain tree sectors can result in low species richness, with only specific species adapted to exploit those resources effectively. Consequently, certain sectors may be underutilized by birds, while others may show a higher concentration of bird activity and resource utilization. In our study, an important consideration that was not accounted for is the vitality of the trees, which could significantly influence bird populations (Villard and Foppen, 2018). Consequently, to draw more conclusive findings, additional research is warranted.
So far, the value of individual tree species for birds in BNP has been assessed concerning nesting site characterization, while our study shows additional aspects related to the use of trees by birds, showing a wider aspect of niche partitioning. We determine that birds are the least specialized in the middle canopy, as such space could offer them easily accessible resources. Additionally, we create increasing niche partitioning in this tree space. Outer parts of the tree and trunks could represent habitat filters, reducing bird diversity, but at the same time generating high species specializations.
We confirm the hypothesis of tree species' importance in forest space sharing among bird assemblage. We found that hornbeam, alder, and spruce are universal species and very widely, almost entirely, are used by birds. They seem to be foundation species, that could generate large partitioning, that is a lot of species and a lot of narrow niches. Others, such as oak, lime, and maple, are used only in certain parts of the tree. Considering the habitat filtering concept, tree species with less complex structures could shape bird assemblage, as they can offer birds much fewer options. Due to high specialization and niche separation, conserving high bird diversity requires diverse stand structures, offering various tree species and sectors, therefore maintaining the natural spatial structure of the forest is important.
This research was conducted in compliance with Polish law and Białowieża National Park regulations.
Oliwia Karpińska: conceptualization, methodology, investigation, formal analysis, visualization, writing – original draft, writing – review and editing. Katarzyna Kamionka-Kanclerska: conceptualization, investigation, methodology, writing - review and editing. Marcin Dyderski: conceptualization, writing - review and editing. Patryk Czortek: conceptualization, formal analysis, visualization, writing - review and editing. Dorota Czeszczewik: conceptualization, methodology, investigation, writing - review and editing, supervision.
Data are available on request from the authors.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We dedicate this work to the memory of the late Wiesław Walankiewicz, who inspired us to research. We are also immensely grateful to Klaudia Karpińska for creating Fig. 2.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fecs.2023.100129.
| [1] |
Adamík, P., Korňan, M., 2004. Foraging ecology of two bark foraging passerine birds in an old-growth temperate forest. Ornis Fenn. 81(1), 13–22.
|
| [2] | |
| [3] | |
| [4] | |
| [5] | |
| [6] | |
| [7] | |
| [8] | |
| [9] | |
| [10] |
Boratyńska, K., 1993. Systematyka i rozmieszczenie geograficzne. In: Bugała, W. (Ed.), Grab Zwyczajny – Carpinus Betulus L. Nasze Drzewa Leśne. Monografie Popularnonaukowe 9, Sorus, Poznań–Kórnik.
|
| [11] | |
| [12] | |
| [13] | |
| [14] |
Dormann, C.F., Gruber, B., Fründ, J., 2008. Introducing the bipartite package: analysing ecological networks. R News 8, 8–11.
|
| [15] | |
| [16] | |
| [17] | |
| [18] |
Faliński, J.B., 1977. Research on vegetation and plant populations dynamics conducted by Białowieża Primeval Forest the Warsaw University in the Białowieża Primeval Forest 1952-1977. Phytocoenosis 6 (1/2), 1–148.
|
| [19] | |
| [20] |
Faliński, J.B., 1986. In: Vegetation dynamics in temperate lowland primeval forest. Ecological studies in Białowieża Forest. Springer, Dordrecht.
|
| [21] |
Faliński, J.B., Hereźniak, J.M., 1977. Zielone grady i czarne bory Białowieży. Instytut Wydawniczy “Nasza Ksiegarnia”, Warszawa.
|
| [22] | |
| [23] | |
| [24] | |
| [25] | |
| [26] |
Hoadley, R.B., 1998. Chemical and physical properties of wood. In: Dardes, K., Rothe, A. (Eds.), The Structural Conservation of Panel Paintings: Proceedings. Part 1: Wood Science and Technology. The Getty Conservation Institute, Los Angeles, pp. 2–20.
|
| [27] | |
| [28] | |
| [29] |
Johnson, O., 2004. Collins Tree Guide. Harper Collins Publishers, London.
|
| [30] | |
| [31] | |
| [32] | |
| [33] | |
| [34] | |
| [35] | |
| [36] | |
| [37] | |
| [38] | |
| [39] | |
| [40] | |
| [41] | |
| [42] |
Pakkala, T., Tiainen, J., Pakkala, H., Piha, M., Kouki, J., 2020. Nest tree characteristics of Grey-headed Woodpeckers (Picus canus) in boreal forests. Ornis Fenn. 97(3), 89–100.
|
| [43] | |
| [44] | |
| [45] | |
| [46] |
R Core Team, 2022. R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
|
| [47] | |
| [48] | |
| [49] | |
| [50] | |
| [51] |
Szmidt, A., 1993. Ważniejsze szkodniki. In: Białobok, S., Boratyński, A., Bugała, W. (Eds.), Biologia Sosny Zwyczajnej. Sourus, Poznań – Kornik, pp. 368–395.
|
| [52] | |
| [53] | |
| [54] | |
| [55] |
Tucker, G.M., Evans, M.I., 1997. Habitats for birds in Europe: a conservation strategy for the wider environment. BirdLife Conservat. Ser. 6, 464. BirdLife International, Cambridge.
|
| [56] | |
| [57] |
Villard, M.A., Foppen, R., 2018. Ecological adaptations of birds to forest environments. In: Mikusiński, G., Roberge, J.M., Fuller, R.J. (Eds.), Ecology and Conservation of Forest Birds. Cambridge University Press, Cambridge, pp. 51–78.
|
| [58] |
Walankiewicz, W., Czeszczewik, D., Mitrus, C., 2007. Natural nest sites of the Collared Flycatcher Ficedula albicollis in lime-hornbeam-oak stands of a primeval forest. Ornis Fenn. 84(4), 155–162.
|
| [59] | |
| [60] | |
| [61] |
Watson, A., Marquiss, M., Summers, R., 2009. Brief report Abundance of crossbills, Siskins and cone-crops. Ornis Fenn. 86, 38–40.
|
| [62] | |
| [63] | |
| [64] |
Wesołowski, T., 2003. Bird community dynamics in a primaeval forest—is interspecific competition important. Ornis Hung. 12(13), 51–62.
|
| [65] | |
| [66] | |
| [67] | |
| [68] | |
| [69] | |
| [70] | |
| [71] | |
| [72] | |
| [73] |
Wesołowski, T., Martin, K., 2018. Tree holes and hole-nesting birds in European and north American forests. In: Mikusiński, G., Roberge, J.M., Fuller, J.R. (Eds.), Ecology and Conservation of Forest Birds. Cambridge University Press, Cambridge, pp. 79–134.
|
| [74] | |
| [75] |
| Response | Variable | X2 | df | P | Pseudo-R2 |
| d′ index | Position from trunk | 22.240 | 2 | <0.001 | 0.229 |
| Position from ground | 10.598 | 5 | 0.059 | ||
| RR index | Position from trunk | 12.215 | 2 | 0.002 | 0.155 |
| Position from ground | 18.592 | 5 | 0.002 | ||
| d′ index | Tree species | 13.315 | 6 | 0.038 | 0.108 |
| RR index | Tree species | 31.151 | 6 | <0.001 | 0.134 |