| Citation: | Vinicio Carrión-Paladines, Ángel Benítez, Roberto García-Ruíz. Conversion of Andean montane forest to exotic forest plantation modifies soil physicochemical properties in the buffer zone of Ecuador's Podocarpus National Park[J]. Forest Ecosystems, 2022, 9(1): 100076. DOI: 10.1016/j.fecs.2022.100076 |
| Abbreviations | |
| AMF | Andean montane forests |
| Bz | buffer zone |
| PNP | the Podocarpus National Park |
| SOM | soil organic matter |
| SOC | soil organic carbon |
| SNAP | the National System of Protected Areas |
| TN | total nitrogen |
| EGP | Eucalyptus globulus plantations |
| PPP | Pinus patula plantations |
Andean montane forests (AMF) are valuable ecosystems that contain high biodiversity and unique levels of endemism (Young and León, 2000; Marian et al., 2020). They are distributed in South America from Venezuela (Jones et al., 2021), Colombia (Rodríguez-Alarcón et al., 2018), Ecuador (Salazar et al., 2020), Peru (Aragón et al., 2021), Bolivia (Kessler, 2001), Chile (Muñoz et al., 2012) to Argentina (Ojeda and Trejo, 2002). AMFs fulfill important ecological functions such as carbon sequestration in vegetation and soil (Malhi et al., 2017), and control and maintenance of water flows during dry periods, making them of singular hydrological importance in the tropics (Bruijnzeel et al., 2006). The high organic matter content in the soil of these forests provides special physical and hydraulic properties, such as low bulk density, high porosity, high infiltration capacity, high water retention capacity, and high hydraulic conductivity (Osorio and Bahamon, 2008; Tobón et al., 2009). These hydrophysical soil properties give these ecosystems a high-water regulation capacity in the watershed where they are located (Tobón et al., 2009).
In Ecuador, most Andean primary forests are part of the National System of Protected Areas (SNAP), in which 20% of all Ecuadorian forests are protected (Kleemann et al., 2022). However, most of the deforestation and subsequent land-use change occurs in unprotected primary forests, which are subsequently degraded (Beck et al., 2008; Marian et al., 2020) and converted to pasture (Günter et al., 2009; Tapia-Armijos et al., 2015) and timber plantations (Weber et al., 2008). According to the IUCN Red List of Species (IUCN-RL), Ecuador's AMFs contain more than twice as many endangered species compared to other Andean countries (Mounce et al., 2018), and are considered amongst the most threatened in the region.
These ecosystems are threatened by hunting and illegal trafficking of timber species (Peyton et al., 1998) because they contain biological diversity characterized by their uniqueness and rarity (Aguirre et al., 2017). However, despite efforts to conserve these ecosystems, such as the establishment of the National System of Protected Areas (SNAP) in buffer zones (Bz), many forests continue to be logged due to conversion processes. In this context, few studies have been conducted to determine the impacts of forest conversion in the Bzs of Ecuador's national parks. Within the Bz of the Podocarpus National Park (PNP), Valarezo-Torres et al. (2021) determined a number of indicators of soil quality/health, while Lozano and Bussmann (2005) studied soil slides caused by human activities and Jacquemin et al. (2012) described how the physicochemical properties of soils have an indirect effect on assemblages of ants living in the soil. For this reason, it is necessary to develop research to understand the impacts of AMF conversion, identifying problems or needs, and thus making the best decisions for the sustainable management of natural resources in the PNP-Bz. In this way, the impacts of anthropogenic activity (conversion) could be minimized, which according to Marian et al. (2020), are leading to reduced diversity and changes in soil characteristics compared to primary forests. Furthermore, Quichimbo et al. (2020) reported that when forests are transformed into crop and grazing areas, they are generally abandoned due to the potential loss of soil quality. These anthropogenic impacts have led government authorities, for some decades, to carry out reforestation projects in degraded areas with exotic species, specifically pine (Pinus patula) and eucalyptus (Eucalyptus globulus), in an attempt to remedy the damage caused by the transformation of native forests (Evans, 2009).
Pine and eucalyptus plantations have been established in the Andean region of Ecuador without consideration of technical and scientific criteria (van Voss et al., 2001). Many farmers and reforestation programs have used them to establish multipurpose artificial plantations for the purposes of timber production, soil erosion prevention, to increase hydrological control, and for the implementation of carbon sequestration pilot projects (Chacón et al., 2009; Knoke et al., 2014). According to Allen et al. (2015), the main effect of this conversion is that soil nutrients are immobilized faster and fewer nutrients are returned to the soil than occurs with native forests. This is because the modification of the vegetation structure can affect the natural processes of the soil nutrient cycle, which has important implications for forest management (Villa et al., 2022). Likewise, it has been shown that different types of soil respond differently to these changes. Crovo et al. (2020) showed that, depending on the soil type, there are changes in the main biogeochemical reservoirs (C, N, and P) between natural deciduous broadleaf forests and pine plantations. Therefore, it is necessary to understand the response of the soil to changes in use that affect physical and chemical properties; such data would serve to better understand the potential impacts of such conversions from natural forests to plantations.
The main studies carried out in Ecuador on the impacts of these plantations on the ecosystems are those developed in the center and north of the country, in areas belonging to the humid tropical alpine environments known as páramos, with special emphasis on edaphic and hydrological aspects (e.g., Farley et al., 2004; Buytaert et al., 2011). For the southern Andean region, where there is a contrast between the humid eastern portion of the Andes, the drier Andean valleys and the western side (FAO, 2000), there is still little knowledge about the impacts of these plantations on natural ecosystems (Quichimbo et al., 2020). For example, in southern Ecuador, classifications of forest sites for pine plantations have been carried out, and litter production and nutrient dynamics have been studied taking into consideration some physicochemical properties of the soil (Quichimbo et al., 2017, 2020; Marian et al., 2020). In the case of eucalyptus, Chacón et al. (2015) found that these types of plantations increase the pH value, although Fe concentrations are lower compared to corn (Zea mays L.), native forest and Pinus patula plantations. In addition, Iglesias Abad et al. (2018) found that the use of eucalyptus biomass (biochar) serves to improve soil conditions and therefore improves the productive yield of corn crops. According to Hofstede et al. (2002), this type of plantation does not produce negative and irreversible impacts on the soil, although they identified some trends that show that the soils under this type of plantation are drier and less organic. Other studies, such as the one by Balthazar et al. (2015), showed that the conversion of degraded agricultural land to pine plantations led to better ecological conditions, although, many times, they are not economically profitable (Leischner and Bussmann, 2003). In this context, more studies are still needed to know and understand the effects of exotic plantations on soil quality/health, which is a challenge for researchers in southern Ecuador (Günter et al., 2009).
We hypothesized that by transforming the AMFs into pine or eucalyptus forests, the stocks of soil carbon and nutrients and the role of AMFs as water regime regulators significantly decrease. To test this hypothesis, we analyzed the main physicochemical properties of the soils in pine and eucalyptus plantations planted in the Bz of the PNP and compared with that of AMFs of southern Ecuador. The results of this research could help decision-makers to identify highly degraded ecosystems in the Bz of the PNP, where appropriate policies and sustainable environmental management should be implemented.
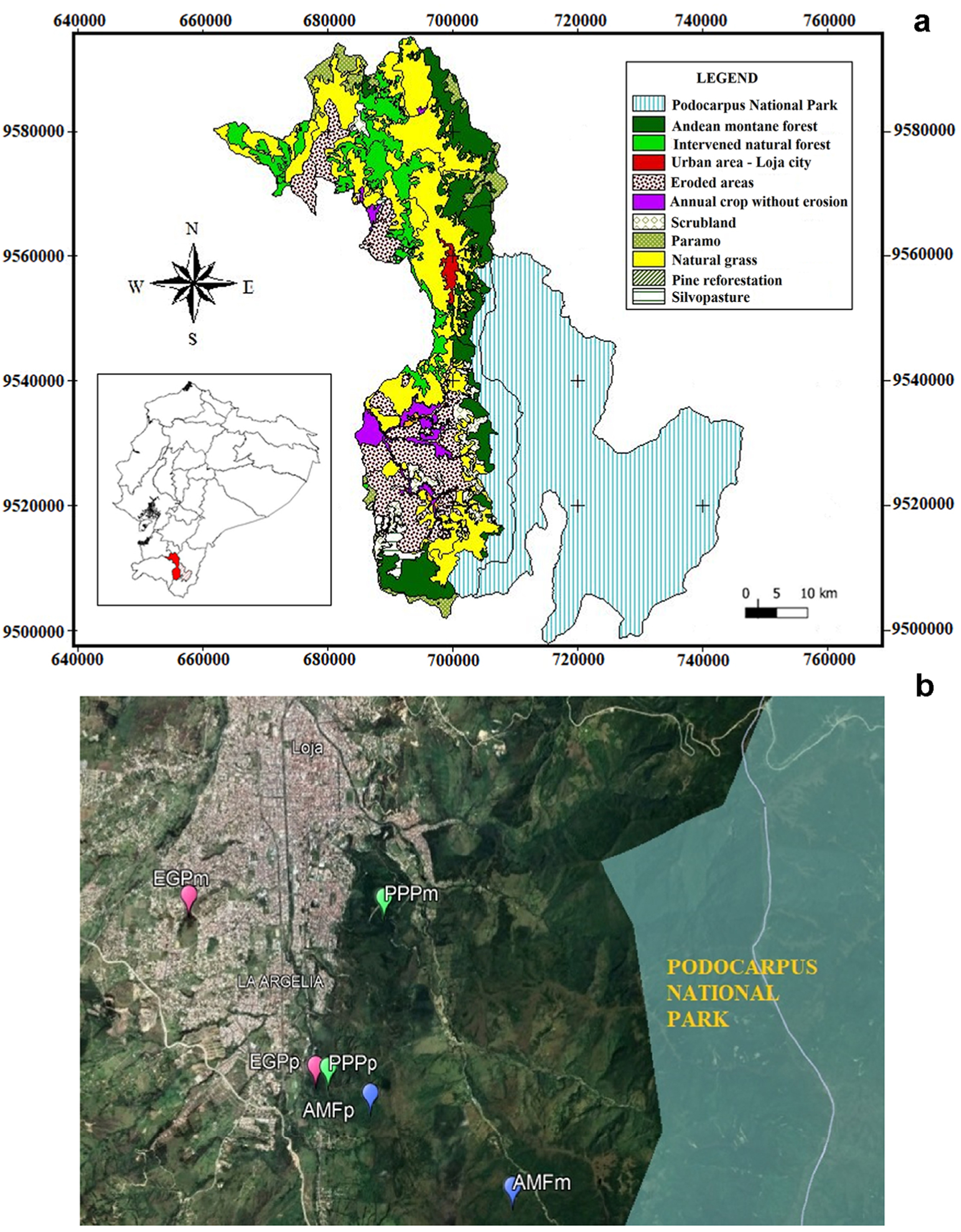
The research was conducted in the province, Canton and city of Loja (southern Ecuador: 3°59′50.25″ S and 79°12′23.46″ W) in the northwestern Bz of the Podocarpus National Park (Fig. 1). The climate in the study area is temperate-equatorial subhumid, with a mean annual rainfall of 1,123.8 mm and a mean annual temperature of 16.4 ℃ (Ochoa-Jiménez et al., 2015; Valarezo Torres et al., 2021). Precipitation presents interannual variability with a minimum mean of 752.7 mm and a maximum of 1,848.1 mm (INAMHI period 2004–2013; La Argelia M0033 meteorological station) and a clear annual cycle with a rainy season from October to April (austral summer) that accounts for more than 75% of the annual rainfall, and a dry season from May to September (austral winter) (Fries et al., 2020) (Fig. 2a and b). The ecosystem is classified as Andean montane forest (AMF) with the presence of herbaceous páramo at higher elevations (Aguirre et al., 2017; Bernátková et al., 2021). The area has a complex topography, due to ongoing orogenic processes, which created abrupt changes between valleys and mountain ranges (Fries et al., 2020), and belongs to the Dystrudepts and Hapludolls soil taxonomic classification, with subgroups within Andic Dystrudepts and Andic Hapludolls that are characterized by having a pH ranging between values of 4.8 and 5.8, considered to be very strongly acidic and a sandy loam texture (Soil Survey Staff, 2014).
The study area contains a wide variety of land use classes, such as pine (Pinus patula) and eucalyptus (Eucalyptus globulus) forest plantations, grazing areas (Pennisetum clandestinum Hoechst Ex Chiov.; Lolium perenne L.), as well as areas of agricultural polycultures mainly consisting of chard (Beta vulgaris L.), tomato (Solanum lycopersicum L.), broccoli (Brassica oleracea L.), potato (Solanum phureja Juz. Et Buk.), among others (Valarezo Torres et al., 2021). The economy of the local population is based mainly on subsistence agriculture and livestock farming (Raes et al., 2017).
Six sites of the buffer zones (Bz) of the Podocarpus National Park (PNP) were selected according to land uses. Two sites were selected in the Andean montane forests (AMFm and AMFp, hereafter), two in eucalyptus plantations (Eucalyptus globulus) (EGPm and EGPp, hereafter), and two in pine plantations (Pinus patula) (PPPm and PPPp, hereafter) (Table 1). The identification of each contrasted site was determined through field excursions and by using the Google Earth Pro program (Del Rio et al., 2018). The distances (km) from the PNP limit parallel to the location of each study site (east-west direction) was determined for each site. The mean altitude of each site was determined with a GPS (Garmin eTrex Legend) and the mean slope of the terrain with a clinometer (Sultan et al., 2017). The slope of the selected sites ranged between 20% and 30%.
| Land use | Sites | Geographical coordinates | Altitude (m a.s.l.) | Terrain slope (%) | Distance from PNP boundary (km) |
| Andean montane forest (AMFm) | Ecological reserve El Madrigal | 4°2′53.27″ S, 79°10′22.37″ W | 2443 | 25 | 1.5 |
| Andean montane forest (AMFp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′18.39″ S, 79°11′26.80″ W | 2365 | 30 | 3.9 |
| Eucalytus plantations (EGPm) | Mater Dei, Loja | 4°0′59.32″ S, 79°12′53.94″ W | 2202 | 20 | 6.1 |
| Eucalytus plantations (EGPp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′8.31″ S, 79°11′53.22″ W | 2192 | 20 | 4.7 |
| Pine plantations (PPPp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′8.64″ S, 79°11′46.82″ W | 2260 | 30 | 4.5 |
| Pine plantations (PPPm) | Forest plantation, Loja | 4°0′56.46″ S, 79°11′27.17″ W | 2190 | 25 | 3.8 |
The AMFm area belongs to the 800-ha private reserve of El Madrigal and is the closest to the PNP with elevations ranging from 2,200 to 3,300 m a.s.l. (Bernátková et al., 2021). The AMFm reserve is in a pristine state of conservation and is inhabited by Andean bears (Tremarctos ornatus) (Cisneros-Vidal, 2013). The main forest species are Cedrela montana Moritz ex Turcz., Clusia alata Planch. & Triana, Oreopanax rosei Harms, Oreopanax andreanus Marchal, Weinmannia glabra L.f., Roupala loxensis I.M. Johnst., Vismia baccifera (L.) Triana & Planch., Palicourea amethystina (Ruíz & Pav.) DC. and Phenax laevigatus Wedd. The second zone of AMFs (AMFp) is a forest of the Francisco Vivar Castro University Park (belonging to the National University of Loja). AMFp covers an area of 99.13 ha, altitude range from 2,130 to 2,520 m a.s.l. and is used as an area for university practices and environmental recreation (Aguirre et al., 2016). It is characterized by having important forest species such as the cascarilla or “quina” (Cinchona officinalis L.), Cedrela montana Moritz ex Turcz., Oreopanax andreanus Marchal, Oreopanax rosei Harms, Clusia alata Planch. & Triana, Alnus acuminata Kunth, Palicourea amethystina (Ruíz & Pav.) DC., and Phenax laevigatus Wedd. (Aguirre et al., 2017). The forest plantations correspond to eucalyptus (Eucalyptus globulus, EGPm and EGPp, respectively) and pine (Pinus patula, PPPp and PPPm, respectively) planted 30 years ago, each to fulfill conservation and recreation functions (PREDESUR, 2004) (Table 1, Fig. 1b).
Soil sampling was carried out between May and September 2021. Four plots of 20 m × 20 m were installed at each study site (1600 m2 in each site, 9600 m2 in total), which were evenly spaced with a distance of 20 m between each one. At each plot, the depth (cm) of the litter layer in the soil was obtained as data related to soil quality (Toledo et al., 2014). This was determined in 4 randomly selected sites per plot by inserting a knife into the mineral soil layer and then measuring with a tape measure (Bergemann and Largent, 2000). In three randomly selected sites of each of the plots, two top 10-cm soil samples were randomly taken within each plot using standardized metal cores (6-cm diameter, 10-cm height, 283-cm3 volume) (Carrión-Paladines et al., 2021). One of the top 10-cm soil samples was used to calculate bulk density (Bd, g·cm−3) and porosity (Pr, %), whereas the other was utilized for chemical analysis. This resulted in 6 top 10-cm soil samples per plot; 3 for Bd-porosity, 3 for chemical analysis, and 12 in total. The samples for chemical analysis were mixed to obtain one composite sample per plot (4 composite samples per zone and 24 soil samples in total). Soil samples were packed in separate plastic bags, labeled, and transported during the same day of collection to the soil laboratory of the Universidad Técnica Particular de Loja (UTPL). Once in the laboratory, samples were air-dried at room temperature for 10 d for subsequent analysis.
In the laboratory, Bd was first determined by the cylinder method (Sandoval et al., 2011), for which individual Bd samples were dried in an oven for 48 h at 105 ℃. Total soil porosity was determined using the assumption of a soil particle density of 2.65 g·cm−3 (Mingming et al., 2018). Samples for the chemical properties were sieved through a 2-mm mesh. Soil texture was determined using the bouyoucos hydrometer method (Black et al., 1965), while soil pH was measured with a pH-meter applying the standard method (Black et al., 1965). Soil organic matter (SOM) was determined using the Walkley & Black method (Page et al., 1982), and the organic carbon content (SOC) was estimated using the Van Bemmelen factor of 1.724 based on the assumption that soil organic matter contains 58% Carbon (1/0.58 = 1.724) (Vela Correa et al., 2012). Total nitrogen (TN) was determined by the Kjeldahl method which also allowed the calculation of the C/N ratio. Available phosphorus content (mg·kg−1) was determined by the modified Olsen method (Bremner, 1966) and potassium content (cmol·kg−1) was determined by atomic absorption spectrophotometry (Tan et al., 2012).
SOC stocks were calculated using the following equation (Yigini et al., 2018):
SOCi stock (Mg C·ha−1) = OCi × BDi × (1 – vGi) × ti × 0.1
where.
OCi = organic carbon content for depth i (%).
BDi = bulk density for depth i (Mg·m−3),
vGi = stoniness, i.e. volumetric content of the coarse fraction for depth i (%),
ti = horizon thickness i (m).
The data from the physical-chemical soil analysis of the different contrasted areas were subjected to a one-way analysis of variance (ANOVA, F-test, P < 0.05). If the statistical analysis was significant, the means were compared with Tukey's post hoc test for their significance (P < 0.05). A normality test (Shapiro-Wilk) was performed on all mean values before parametric tests were applied.
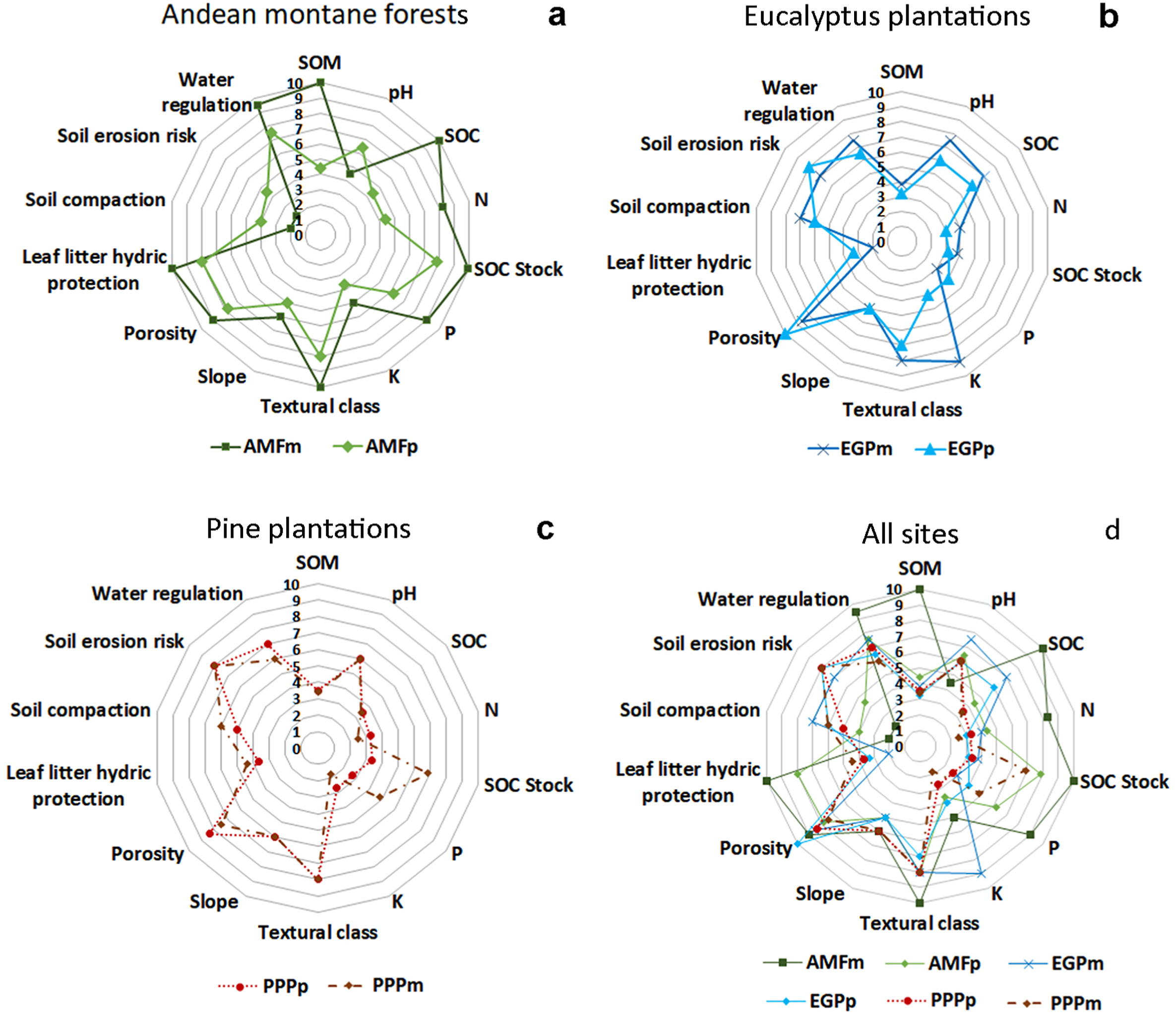
To determine soil quality in terms of water regulation, risk of soil erosion and compaction in each contrasted zone, we used the methodology of Levi et al. (2021), who propose the use of soil physicochemical properties and leaf litter depth as indicators of soil quality. For this purpose, a systematization of these indicators was carried out, categorizing each parameter according to their optimal levels and concentrations considered in the specialized literature, such as deficient, normal, and high (e.g., Meza Aliaga et al., 2017). The scale used was evaluated as 1 = low, 5 = medium, and 10 = high. With these data, it was possible to construct the respective spider diagrams that have been used in previous studies (e.g., Langeveld et al., 2012). In addition, to interpret the combined impacts between the contrasting zones (AMFm, AMFp, EGPm, EGPp, PPPp and PPPm) with the effects of biotic factors (leaf litter depth), abiotic factors (altitude), and changes in soil properties, a principal component analysis (PCA) was performed. Factor correlation analysis was first performed before PCA. Analysis of variance (ANOVA) was performed using SPSS statistical software (SPSS Inc., v.15.0; Chicago, IL, USA) and spider diagrams were prepared with Excel MS Office (Microsoft, USA) (Pogrzeba et al., 2018). Principal component analysis (PCA) was performed using PAST version 3 software (Hammer et al., 2001).
Table 2 shows the main physical properties of the soils and litter depth (cm) in the different contrasting zones. Bulk density (Bd) ranges from 0.37 g·cm−3 in the pristine AMFm to 0.76 g·cm−3 in the anthropized PPPm presenting significant statistical differences (P < 0.05). It is important to note that there are significant differences between native forests (AMFm, 0.37 g·cm−3; and AMFp, 0.67 g·cm−3, P < 0.05) and specifically AMFm differs from all other areas studied (EGPm, 0.68 g·cm−3; EGPp, 0.53 g·cm−3; PPPp, 0.49 g·cm−3; PPPm, 0.76 g·cm−3). This same pattern was observed for both soil porosity (AMFm 86.5%–71.7% in anthropized PPPm) and litter depth (AMFm 27.8 to 4.8 cm in anthropized EGPm).
| Andean montane forests and forest plantations | Bulk density (g·cm−3) | Porosity (%) | Leaf litter depth (cm) | Sand (%) | Silt (%) | Clay (%) | Textural class |
| AMFm | 0.37 ± 0.1 a | 86.5 ± 3.8 a | 27.8 ± 2.2 a | 87.6 ± 3.2 a | 7.7 ± 1.3 a | 4.8 ± 2.3 a | Sandy ground |
| AMFp | 0.67 ± 0.1 b | 74.6 ± 4.2 b | 17.0 ± 0.8 b | 76.6 ± 7.3 b | 11.9 ± 10.3 a | 11.5 ± 5.7 b | Sandy loam |
| EGPm | 0.68 ± 0.1 b | 74.0 ± 3.0 b | 4.8 ± 1.1 c | 74.8 ± 6.2 b | 10.1 ± 3.6 a | 15.1 ± 3.8 b | Sandy loam |
| EGPp | 0.53 ± 0.1 c | 80.1 ± 3.7 c | 9.3 ± 3.4 d | 64.6 ± 8.3 c | 12.4 ± 6.6 a | 23.0 ± 8.4 c | Sandy clay loam |
| PPPp | 0.49 ± 0.2 c | 81.0 ± 8.8 c | 10.5 ± 1.3 d | 70.4 ± 5.5 ab | 13.8 ± 3.7 a | 15.9 ± 3.5 b | Sandy loam |
| PPPm | 0.76 ± 0.2 d | 71.7 ± 6.6 d | 12.3 ± 1.3 d | 71.9 ± 7.7 ab | 13.5 ± 3.7 a | 14.6 ± 5.0 b | Sandy loam |
On the other hand, the soils have a sandy ground (AMFm), sandy loam (AMFp, EGPm, PPPp and PPPm) and sandy clay loam texture (EGPp) (Table 2).
Table 3 presents the main chemical properties of the soils of each contrast zone (depth 0–10 cm). There are significant statistical differences between the studied zones (P < 0.05), where the pristine forest (AMFm) is statistically different from all the contrast zones. For example, in AMFm the soil pH (3.2) differs even from the other intervened natural forest (AMFp, pH 4.5) and from the rest of the contrasting zones (EGPm, pH 4.7; EGPp, pH 4.1; PPPp, pH 4.2; and PPPm, pH 4.0, respectively). Likewise, in terms of SOM content, AMFm (20.5%) differs from the other contrasting zones such as AMFp (9.0%), EGPm (7.8%), EGPp (6.6%), PPPp (7.2%) and PPPm (4.0%). The same occurs with the contents of total nitrogen (TN), and soil organic carbon (SOC), SOC stock, C/N ratio, phosphorus, and potassium, which maintain this trend.
| Montane forests and forest plantations | pH | SOM (%) | TN (%) | SOC (%) | SOC stock (Mg SOC·ha−1) | C/N ratio | P (mg·kg−1) | K (cmol·kg−1) |
| AMFm | 3.2 ± 0.2 a | 20.5 ± 2.9 a | 1.0 ± 0.1 a | 11.9 ± 1.7 a | 39.6 ± 5.6 a | 11.9 ± 0.0 a | 23.0 ± 11.2 a | 0.4 ± 0.2 a |
| AMFp | 4.5 ± 0.4 bc | 9.0 ± 1.3 b | 0.5 ± 0.1 b | 5.2 ± 0.7 b | 31.6 ± 4.4 a | 10.4 ± 0.1 a | 15.8 ± 2.9 ab | 0.3 ± 0.0 a |
| EGPm | 4.7 ± 0.2 c | 7.8 ± 2.4 b | 0.4 ± 0.1 b | 4.5 ± 1.4 b | 27.8 ± 8.4 b | 11.3 ± 0.0 a | 7.3 ± 3.0 b | 1.0 ± 0.1 b |
| EGPp | 4.1 ± 0.3 bc | 6.6 ± 0.7 b | 0.3 ± 0.0 b | 3.8 ± 0.4 b | 18.2 ± 1.8 c | 12.6 ± 0.0 a | 9.4 ± 3.1 b | 0.4 ± 0.1 a |
| PPPp | 4.2 ± 0.3 bc | 7.2 ± 1.5 b | 0.4 ± 0.1 b | 4.2 ± 0.9 b | 13.3 ± 3.9 c | 10.5 ± 0.1 a | 6.9 ± 3.4 b | 0.3 ± 0.1 a |
| PPPm | 4.0 ± 0.2 b | 6.9 ± 1.9 b | 0.3 ± 0.1 b | 4.0 ± 0.1 b | 27.3 ± 7.6 b | 13.3 ± 0.1 a | 12.3 ± 1.3 ab | 0.2 ± 0.0 a |
Fig. 3 shows the evaluation of soil water regulation capacity, erosion risk, and soil compaction risk for each zone studied. The AMFm has the best water regulation capacity and almost no risk soil erosion and compaction, followed by AMFp (Fig. 3a). However, it is observed that for both eucalyptus (Fig. 3b) and pine plantations (Fig. 3c) there is a lower water regulation capacity and a higher risk of soil erosion and compaction. Fig. 3d shows the spider diagram considering all the sites in this study.
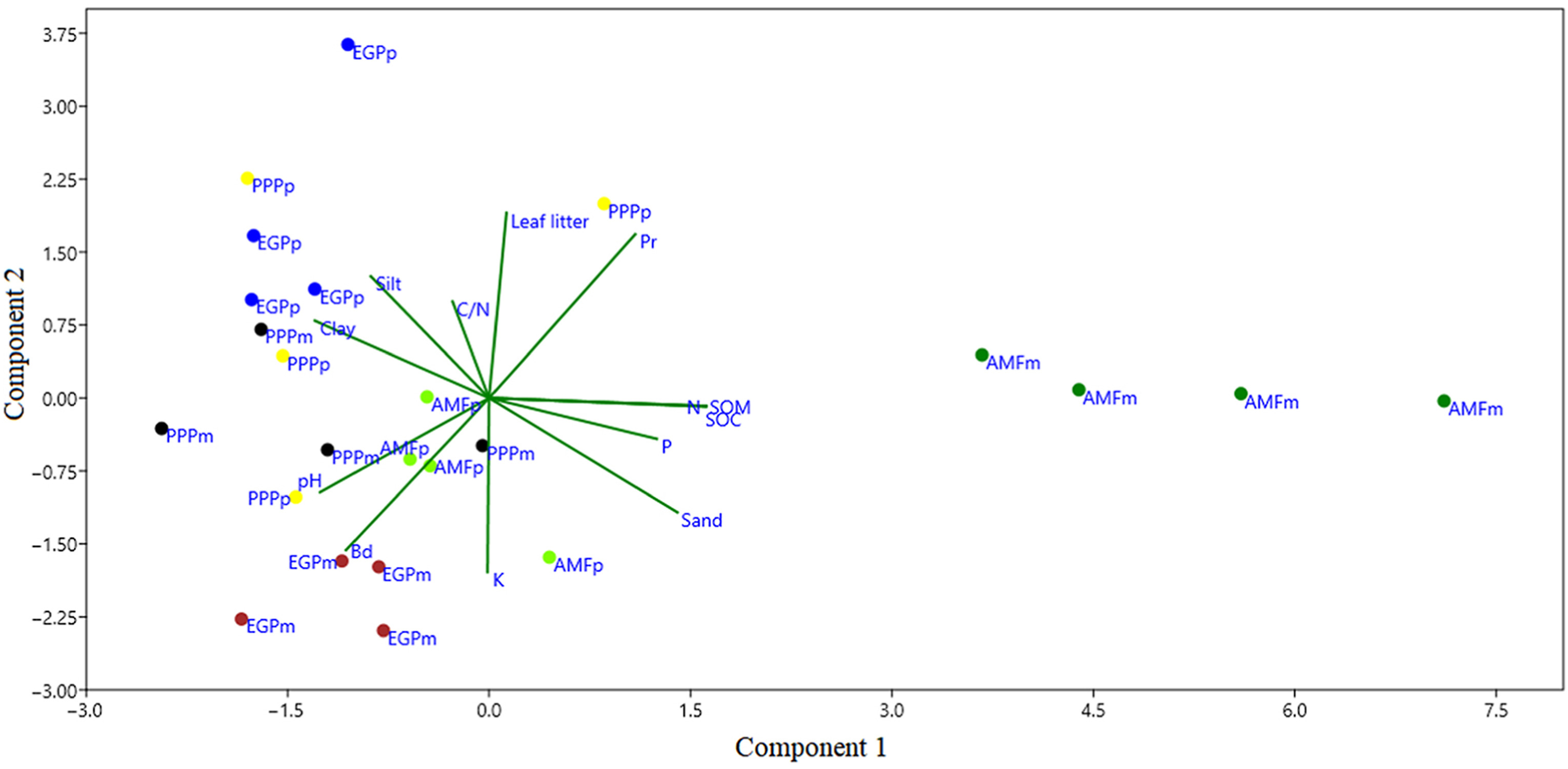
Fig. 4 presents the results of the PCA between the contrasting zones considering the physical-chemical properties of the soil and some environmental attributes. Component 1 and 2 account for 53.1% and 13.9% of the variance, respectively. The AMFm was significantly different from all other contrast zones, including the anthropized AMFp. Soils of the AMFm were positively correlated with variables related to soil water holding capacities, such as higher litter depth, SOM, N, C, lower Bd and higher porosity, as well as N and C contents. In addition, AMFm was negatively related to Bd (where there is greater soil compaction), clay, silt, and pH contents. The rest of the contrasting (anthropized) zones have similarities within the variables studied.
The results obtained in this study are consistent with those reported by other research that has shown that the conversion of AMFs to pine and eucalyptus plantations affects the natural functioning of biogeochemical cycles and the water regulation capacity of soils (e.g., Farley et al., 2004; Balthazar et al., 2015). Among soil physical properties, for example bulk density (Bd), our results are similar to those reported by Camenzind et al. (2016), who also found low Bd values in AMFs from southern Ecuador (range 0.11–0.91 g·cm−3). In this context, AMFm differs from all other contrasting zones in the study area (even with AMFp), presenting the lowest Bd value and therefore presents the best conditions for avoiding soil compaction (AMFm, 0.37 g·cm−3; AMFp, 0.67 g·cm−3; EGPm, 0.68 g·cm−3; EGPp, 0.53 g·cm−3; PPPp, 0.49 g·cm−3 and PPPm, 0.76 g·cm−3 respectively). According to studies by Llerena Pinto (2018), this difference is because within the AMFm there are high inputs of organic matter as a result of the existent abundant biodiversity, which in turn produces large amounts of leaf litter in the soil (AMFm, 27.8 cm; AMFp, 17.0 cm; EGPm, 4.8 cm; EGPp, 9.3 cm; PPPp, 10.5 cm; and PPPm, 12.3 cm deep, respectively), providing for the best conditions to maintain low Bd values and improved soil porosity. Therefore, Herzog et al. (2011) in their studies demonstrated the positive effect of leaf litter on the soil, which fulfills the function of reducing the impact of raindrops and thus interrupting the flow of surface water (minimizing kinetic energy) thereby preventing erosion and soil compaction (Doornbos, 2015). In this context, even the AMFm has a low Bd in comparison to the soils of the other AMFp (anthropized), likely due to the former being a pristine forest, and differing from the latter which is a forest where university practices and recreational excursions of the population of Loja take place. The same happens with the AMFm which is totally different from the eucalyptus and pine plantations (EGPm, EGPp, PPPp and PPPm) (Fig. 3 and 4). Although AMFm has the lowest Bd, highest soil porosity, and the best water regulating function, the pine and eucalyptus plantations, although not optimal, also fulfill this hydrophysical function. In plantations, Bd values were higher (range from 0.49 to 0.76 g·cm−3, Table 2), but in EGPm, EGPp, PPPp, and PPPm there is also litter cover (4.8, 9.3, 10.5 and 12.3 cm deep, respectively), thus protecting the soil environment (avoiding erosive events) and helping to improve biological and physicochemical properties, as reported by recent studies (Zhu et al., 2020). Perhaps the difference between the AMFm and the studied plantations is due to a greater human presence. After all, they are frequently visited for recreational purposes and few silvicultural activities (pruning) are applied.
On the other hand, another reason to conserve pristine AMFs is that the texture of the soil is sandy, and therefore, according to Ligonja and Shrestha (2013), these soils would be highly susceptible to water erosion (Table 2). As such, a change in the landscape use of the AMFm would cause a relatively rapid loss of soil quality due to runoff, since annual rainfall could reach 1,800 mm·year−1 (Fig. 2a). In addition, the exposure of the soil to the elements, on slopes of up to 30% with high seasonal rainfall amounts (Fig. 2b), combined with wind, can cause the soil to lose sand content as an effect of intense erosion, as shown by previous studies (Giertz et al., 2005; Tellen and Yerima, 2018). In the case of the AMFp and the eucalyptus and pine plantations where there is greater human activity, our results are consistent with those reported by Valarezo Torres et al. (2021) who found that in the disturbed montane forests of the Bz of the PNP (areas adjacent to those of this study), the textures of the soil are sandy loam, like those found in the AMFp, EGPm, PPPp and PPPm. Perhaps due to the conversion to this type of plantation, the loss of soil quality occurs, so that over the years (time) and by the processes of natural succession, they present sandy loam and clay loam textures. Consequently, the soils will have mild to moderate susceptibility to water erosion according to the scale described by Ligonja and Shrestha (2013) (Table 2).
Regarding chemical properties, the pH value of AMFm (pH 3.2) is consistent with that reported by Lozano et al. (2010) and Jacquemin et al. (2012) who found average pH values of 3.71 in AMFs close to the PNP. In the case of the most anthropized AMFp (pH 4.5) and forest plantations (EGPm, pH 4.7; EGPp, pH 4.1; PPPp, pH 4.2 and PPPm, pH 4.0), they are consistent with data reported by Chacón et al. (2015), who found pH values of 5.5 for eucalyptus and 4.9 for pine in plantations in southern Ecuador. In these anthropized forests, soil pH was significantly higher concerning AMFm. This condition of soil acidity in AMFm produces effects on the biogeochemistry of the soil. For example, Bueis et al. (2018) showed that the activity of the dehydrogenase enzyme is fifteen times lower than those soils where the pH is close to neutrality. Therefore, the action of this enzyme decreases and could produces a decrease in the metabolic activities of microorganisms (Das and Varma, 2010) and in turn, there would be a low soil microbial mineralization activity, even reaching nutrients immobilization for plants (e.g., Dantas de Paula et al., 2021) (Table 3). However, for tropical forests, other types of enzymes have been studied and used to determine soil quality/health. For example, Turner, (2010) found in tropical rainforest soil of Panama that acid phosphatase activities with a pH optimum below <4 (as in the case of AMFm) partially explained the relatively high values of available P in the soil. This result agrees with those observed by Tripathi et al. (2007) who reported that this enzyme is involved in the hydrolysis of various organic and inorganic phosphate esters in the phosphorus cycle and are synthesized and secreted extracellularly by bacteria or fungi, which are part of the soil matrix. In this context, the high SOM content observed in AMFm and the high P concentrations (23.0 mg·kg−1) could be because most trees in the AMFm form mycorrhizal associations with fungi and these can increase nutrient availability by releasing organic acids that accelerate both weathering and the decomposition of organic matter, as demonstrated in recent research (e.g., Schoonover and Crim, 2015; Mendoza et al., 2022). Likewise, Effron et al. (2006) demonstrated that high SOM content in forests would originate an increase in microbial activity, which is manifested in higher CO2 emissions and would produce an increase in enzyme production and, consequently (at optimum pH), in the decomposition of organic matter (Saetre and Bååth, 2000). Therefore, the results of this study would indicate that the microbial biomass of the AMFm is greater than that found in the AMFp and in pine and eucalyptus plantations, where it is reduced by factors inherent to the management of these systems as has been demonstrated in various investigations (Durángo et al., 2015; Mendoza et al., 2022). In this context, it is suggested to use the analysis of phosphatase enzymes and others as indicators of soil quality/health comparing pristine forests versus exotic forest plantations in the Bz of the PNP. Also, exchangeable potassium was very low in all soils and there was not clear trend in the conversion of AMFm to forest plantation. The lack of differences could be due to the very low K contents of these ancient soils that have been subjected to relatively high annual rainfall for thousands of years, resulting in the depletion of most cations, such as potassium.
On the other hand, soil organic matter (SOM) values for both the AMFm and the rest of the anthropized zones are good (20% for the AMFm and 5%–10% for the anthropized zones). In this sense, all the contrasting zones, except the AMFm, are within the values considered optimal for agriculture (Molina, 2007). However, the high SOM concentration in the AMFm has another treatment or ecological function, as we explain in the section below (Section 4.2). In this context, as explained above, the high concentration of SOM in AMFm is related to the high biodiversity, producing an overaccumulation that would cause an increase in microbial activity and the presence of specific enzymes for biodegradation. This would cause a buffering effect on the pH not to be produced due to the high content of organic matter in AMFm. In addition, this over accumulation is explained by the very humid climatic conditions (1,123.8 mm·year−1) and the temperate temperature of the area (16.4 ℃), which cause slower organic matter transformation processes as reported in other studies in southern Ecuador (Fries et al., 2009; Carrión-Paladines et al., 2021). Similarly, it is known that air temperature generally decreases with altitude in the troposphere and AMFm is the studied area at the highest altitude (2,443 m a.s.l.; Table 1), potentially influencing the slow decomposition of organic matter. This coincides with other areas close to this study, where it has been shown that air temperature also depends on the conversion of native forests to other uses. For example, Carrión-Paladines et al. (2021) determined that more disturbed sites showed higher temperatures compared to native vegetation at the same or even higher altitudes. This effect is caused by the lack of a dense canopy layer (Fries et al., 2009) in disturbed areas. The canopy layer is known to protect the air within the forest from temperature extremes, resulting in generally lower mean temperatures (about 1 ℃) within forest stands compared to disturbed sites. In these areas, it has been determined that in sunny weather conditions, typical of the dry season, the differences are even greater (around 3 ℃), a pattern also observed with air humidity, which is notably higher inside native forests compared to open sites (Fries et al., 2012). In addition, due to the effect of orographic precipitation, rainfall increases towards the higher elevations (Fries et al., 2014), so there is more precipitation in the AMFm, leading to the expectation that organic matter degradation processes in this area are lower but effective compared to the other contrasting areas.
Finally, although the conversion to exotic plantations produces negative changes in the soil physicochemical properties when compared to pristine AMF, recent studies have shown that the soil quality and fertility of these forest plantations are higher than that of other conversion alternatives such as cropland and pasture (e.g., Ahmed et al., 2022). In this context, in our study area, it was determined that in the conversion of pristine AMF in EGPm and EGPp, there is a loss of SOC of 37.8% and 31.9% respectively, and in PPPp and PPPm there is a loss of 35.3% and 33.6% respectively (Table 3). This is consistent with that reported by Guo and Gifford (2002) who reported a higher SOC loss of 42% and 59% in cropland and grassland soil following the conversion of natural forests in Australia, Brazil, New Zealand, and the U.S. Similarly, Wei et al. observed that the conversion of forests to cropland resulted in a decrease in soil SOC storage of 52%, 41% and 31% in temperate, tropical and boreal regions, respectively. Likewise, Abegaz et al. (2020) found a 65% loss of SOC stocks due to the conversion of virgin forests to intensive cropland along a land use chronosequence in the Ethiopian highlands. These losses are attributed to (ⅰ) lack of organic matter inputs through litterfall, dead wood, roots, microbes, and mycorrhizal turnover that exist in forest ecosystems; (ⅱ) very limited inputs of litterfall from crop and pasture residues, as most of the aboveground biomass is grazed or used as fuel; (ⅲ) erosion and removal of topsoil, accelerated by tillage and overgrazing; and (ⅳ) depletion of soil organic matter exposed by oxen plowing up to 30-cm soil depth, which was originally protected from microbial attack (Assefa et al., 2017). Therefore, although exotic plantations do not present the best edaphic conditions, with the application of appropriate management strategies, such as the implementation of mixed plantations (e.g., incorporating nitrifying species in combination with eucalyptus), the establishment of soil conservation works among others, the quality and health of these soils in the Bz of the PNP could be improved.
The high SOM content observed in AMFm (20.5%) is not necessarily of negative consequence as it has been shown that high SOM concentrations allow soils to store a large amount of water, which is very beneficial due to the hydrological function that this AMFm fulfills for water supply (Jacquemin et al., 2012). Therefore, our results coincide with studies in other AMFm, where it has been determined that the higher the SOM content in the soil, the better its structure, becoming more granular, thus favoring the development of a dense and deep root system and generating a greater infiltration and water storage capacity (Tobón et al., 2009; Mogrovejo and Márquez, 2017). Thus, this allows for the recharge of soils and aquifers in these ecosystems to be greater, which means that river flows are maintained even during dry months (Tobón, 2009). In addition, the high SOM content in AMFm soils confers special physical and hydraulic properties, such as low Bd and high porosity (Table 3). These physical conditions allow the soil to have a high infiltration capacity, high moisture retention capacity, and high hydraulic conductivity (Osorio and Bahamon, 2008; Tobón et al., 2009). In addition, Avendaño (2007) determined that this type of soil has a thick layer of mosses and humified matter that has an important effect on the hydrology of these ecosystems, which is why they are capable of storing large quantities of water (up to six times their dry weight). Likewise, the frequency of fog and low evapotranspiration losses in high Andean forests are, in part, responsible for the soils maintaining a high permanent humidity (Schawe et al., 2008; Osorio and Bahamon, 2008). This means that the water yield of these ecosystems is generally higher than that of other forest types and therefore they offer a high water regulation capacity in the watershed where they are located (Tobón et al., 2009). Therefore, the AMFs that are in good conservation status in the Bz of the PNP, need their protection sustained, as they are indispensable for the flow of streams and rivers.
SOM in pine and eucalyptus plantations was significantly lower, typically less than half that of pristine AMFm. According to Farley et al. (2004), when these conversions are carried out to obtain pine plantations, the water-holding capacity of the soils is often altered. This is confirmed by the results of this study, which shows that AMF conversion produces greater reductions in SOM content, higher Bd, lower litter depth, and lower porosity, which could affect water retention as reported in other studies (e.g., Álvarez-Garretón et al., 2019). In addition, Alameda et al. (2012) found that soils under pine plantations lose moisture rapidly after a rain event, which further increases soil desiccation and organic matter degradation. As for eucalyptus plantations, recent studies conducted on afforestation with this species report that during the growth stage, they could play a key role in decreasing SOM (Bonnesoeur et al., 2019), compared to the pristine AMFm site. Generally, these species used for afforestation projects, during their growth stage, have a high water demand and therefore reduce soil water content (Patiño et al., 2021). The main process that occurs under these conditions is that the conversion of AMF to this type of exotic plantation, causes the SOM to be exposed to microbial attack and climatic conditions that favor chemical-physical weathering, and then the SOM decomposition process is accelerated. Moreover, this changes soil conditions, such as pore size distribution, favoring SOM mineralization (aggravating soil condition), which ultimately decreases water holding capacity. Therefore, this confirms our findings and the findings of Farley et al. (2004), as we demonstrate that afforestation with exotic species does not favor soil aeration, but tends to increase Bd (decreased aeration and increased compaction), which is probably the result of decreased SOM as shown in Table 3.
On the other hand, the low amount of SOM under eucalyptus and pine plantations indicates that the transformation of pristine AMF can significantly alter the precipitation-infiltration-runoff relationship as reported by previous research (Marín et al., 2018). This seriously endangers the capacity of these ecosystems as water regulators and therefore the sustainability of clean water supplies for human populations can be affected (Pacheco and Ataroff, 2002). In this context, we recommend promoting political strategies that allow for the continuation of the conservation of these native forests and thus ensure the availability of water for the city of Loja and the populations surrounding the PNP (Fig. 1a and b).
Another aspect that would allow the conservation of AMFs is maintaining carbon reserves in the soil. For example, Barrezueta-Unda et al. (2019) showed that conversion to eucalyptus and pine plantations produces a decrease in soil carbon stocks; so much so that, in southern Ecuador, native forests (36.3 Mg SOC·ha−1) lost considerable rates of carbon when converted to pasture (27.6 Mg SOC·ha−1). In addition, contributions to greenhouse enhancement may occur, as land-use change due to vegetation clearing has been found to influence greenhouse gas emissions (Page and Baird, 2016). IPCC (2001) and Smith (2008) indicated that land-use change emitted 1.6 ± 0.8 Pg C·year−1 to the atmosphere. Therefore, we reiterate that land-use change should be considered in local government regulations, as is the case in the municipality of Loja, to avoid problems due to water depletion, watershed erosion, and greenhouse gas emissions.
In this study, plantation forests present the worst conditions for both water regulation and soil erosion risk (Fig. 3 and 4). This is a threat, even more so when an AMF has been recently cleared for other uses, where catastrophes can occur that can even lead to human fatalities as has already happened in Ecuador (Watson et al., 2022). For example, in the Andes, the well-known “huaycos” can occur, which are frequent landslides (Inbar and Llerena, 2000) with debris avalanches and flash floods, due to heavy rains at AMF heights (Reyes-Knoche, 2012). Therefore, it is recommended that areas near human populations that do not have good water regulation capacity and are susceptible to erosion implement soil conservation works, e.g., slow terracing, afforestation, and construction of sediment trapping dams (Zhang et al., 2008). In addition, recent research has shown that the introduction of nitrogen-fixing trees (e.g., trees of the genus Acacia spp.) in a mixed system with eucalyptus improves the chemical and microbiological properties of the soil, especially on CN contents and microbial diversity (Zagatto et al., 2019). Consideration of these measures will allow for proper management of forest plantations in the Bz of the PNP; however, conservation of the AMF is the most recommended strategy.
This study has identified the effects caused by the conversion of AMF in eucalyptus and pine plantations on the main physicochemical properties of the soil related to quality, water regulation and the maintenance of carbon stocks in the Bz of the PNP. The results show that the main negative effects are related to the loss of SOM, SOC Stock, and increased soil compaction level (higher Bd) in forest plantations. Our results suggest that the change in land use modifies the capacity of water regulation, produces a greater risk of soil erosion and affects the maintenance of carbon reserves, which leads to accelerated processes of degradation as well as a possible scarcity of water supplies. To reduce the negative effects of conversion, soil restoration practices such as the establishment of slow-forming terraces, afforestation, the construction of sediment retention dikes and the introduction of nitrogen-fixing trees of the genus Acacia spp. that will improve the chemical and microbiological properties of the soil are recommended. However, these practices should be carried out with the conservation of pristine AMF as a priority. These findings may help decision makers to identify highly degraded ecosystems in the Bz of the PNP, where appropriate policies and sustainable environmental management should be implemented. In addition, it is recommended that environmental restoration strategies be applied to the anthropized areas of AMFs, as well as areas with eucalyptus and pine plantations.
VCP, ÁB, RGR conceptualization, methodology, validation, investigation, data curation; VCP, RGR analyzed the data; VCP, ÁB, RGR writing–original draft, writing–review and editing.
All the authors have approved the manuscript and agreed with submission to your esteemed journal.
Not applicable.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Our thanks to the Universidad Técnica Particular de Loja for funding this research (PROY_INV_CCBIO_2020_2773). Special thanks to Gregory Gedeon for proofreading the English text.
| Land use | Sites | Geographical coordinates | Altitude (m a.s.l.) | Terrain slope (%) | Distance from PNP boundary (km) |
| Andean montane forest (AMFm) | Ecological reserve El Madrigal | 4°2′53.27″ S, 79°10′22.37″ W | 2443 | 25 | 1.5 |
| Andean montane forest (AMFp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′18.39″ S, 79°11′26.80″ W | 2365 | 30 | 3.9 |
| Eucalytus plantations (EGPm) | Mater Dei, Loja | 4°0′59.32″ S, 79°12′53.94″ W | 2202 | 20 | 6.1 |
| Eucalytus plantations (EGPp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′8.31″ S, 79°11′53.22″ W | 2192 | 20 | 4.7 |
| Pine plantations (PPPp) | Francisco Vivar Castro University Park (PUEAR) | 4°2′8.64″ S, 79°11′46.82″ W | 2260 | 30 | 4.5 |
| Pine plantations (PPPm) | Forest plantation, Loja | 4°0′56.46″ S, 79°11′27.17″ W | 2190 | 25 | 3.8 |
| Andean montane forests and forest plantations | Bulk density (g·cm−3) | Porosity (%) | Leaf litter depth (cm) | Sand (%) | Silt (%) | Clay (%) | Textural class |
| AMFm | 0.37 ± 0.1 a | 86.5 ± 3.8 a | 27.8 ± 2.2 a | 87.6 ± 3.2 a | 7.7 ± 1.3 a | 4.8 ± 2.3 a | Sandy ground |
| AMFp | 0.67 ± 0.1 b | 74.6 ± 4.2 b | 17.0 ± 0.8 b | 76.6 ± 7.3 b | 11.9 ± 10.3 a | 11.5 ± 5.7 b | Sandy loam |
| EGPm | 0.68 ± 0.1 b | 74.0 ± 3.0 b | 4.8 ± 1.1 c | 74.8 ± 6.2 b | 10.1 ± 3.6 a | 15.1 ± 3.8 b | Sandy loam |
| EGPp | 0.53 ± 0.1 c | 80.1 ± 3.7 c | 9.3 ± 3.4 d | 64.6 ± 8.3 c | 12.4 ± 6.6 a | 23.0 ± 8.4 c | Sandy clay loam |
| PPPp | 0.49 ± 0.2 c | 81.0 ± 8.8 c | 10.5 ± 1.3 d | 70.4 ± 5.5 ab | 13.8 ± 3.7 a | 15.9 ± 3.5 b | Sandy loam |
| PPPm | 0.76 ± 0.2 d | 71.7 ± 6.6 d | 12.3 ± 1.3 d | 71.9 ± 7.7 ab | 13.5 ± 3.7 a | 14.6 ± 5.0 b | Sandy loam |
| Montane forests and forest plantations | pH | SOM (%) | TN (%) | SOC (%) | SOC stock (Mg SOC·ha−1) | C/N ratio | P (mg·kg−1) | K (cmol·kg−1) |
| AMFm | 3.2 ± 0.2 a | 20.5 ± 2.9 a | 1.0 ± 0.1 a | 11.9 ± 1.7 a | 39.6 ± 5.6 a | 11.9 ± 0.0 a | 23.0 ± 11.2 a | 0.4 ± 0.2 a |
| AMFp | 4.5 ± 0.4 bc | 9.0 ± 1.3 b | 0.5 ± 0.1 b | 5.2 ± 0.7 b | 31.6 ± 4.4 a | 10.4 ± 0.1 a | 15.8 ± 2.9 ab | 0.3 ± 0.0 a |
| EGPm | 4.7 ± 0.2 c | 7.8 ± 2.4 b | 0.4 ± 0.1 b | 4.5 ± 1.4 b | 27.8 ± 8.4 b | 11.3 ± 0.0 a | 7.3 ± 3.0 b | 1.0 ± 0.1 b |
| EGPp | 4.1 ± 0.3 bc | 6.6 ± 0.7 b | 0.3 ± 0.0 b | 3.8 ± 0.4 b | 18.2 ± 1.8 c | 12.6 ± 0.0 a | 9.4 ± 3.1 b | 0.4 ± 0.1 a |
| PPPp | 4.2 ± 0.3 bc | 7.2 ± 1.5 b | 0.4 ± 0.1 b | 4.2 ± 0.9 b | 13.3 ± 3.9 c | 10.5 ± 0.1 a | 6.9 ± 3.4 b | 0.3 ± 0.1 a |
| PPPm | 4.0 ± 0.2 b | 6.9 ± 1.9 b | 0.3 ± 0.1 b | 4.0 ± 0.1 b | 27.3 ± 7.6 b | 13.3 ± 0.1 a | 12.3 ± 1.3 ab | 0.2 ± 0.0 a |